Ataxia and Disorders of Cerebellar Function: Introduction
The cerebellum is responsible for the coordination of movements, especially skilled voluntary ones, the control of posture and gait, and the regulation of muscular tone. In addition, the cerebellum may play a role in the modulation of the emotional state and some aspects of cognition. The mechanisms by which these functions are accomplished have been the subject of intense investigation by anatomists and physiologists. Their studies have yielded a mass of data, testimony to the complexity of the organization of the cerebellum and its afferent and efferent connections. A coherent picture of cerebellar function is now emerging, although it is not yet possible, with a few exceptions, to relate each of the symptoms of cerebellar disease to a derangement of a discrete anatomic or functional unit of the cerebellum.
Knowledge of cerebellar function has been derived mainly from the study of natural and experimental ablative lesions and to a lesser extent from stimulation of the cerebellum, which actually produces little in the way of movement or alterations of induced movement. Furthermore, none of the motor activities of the cerebellum reaches conscious kinesthetic perception; its main role, a critical one, is to assist in the modulation of willed movements that are generated in the cerebral hemispheres. The following discussion of cerebellar structure and function has, of necessity, been simplified; a fuller account can be found in the writings of Jansen and Brodal, of Gilman, and of Thach and colleagues.
Anatomic and Physiologic Considerations
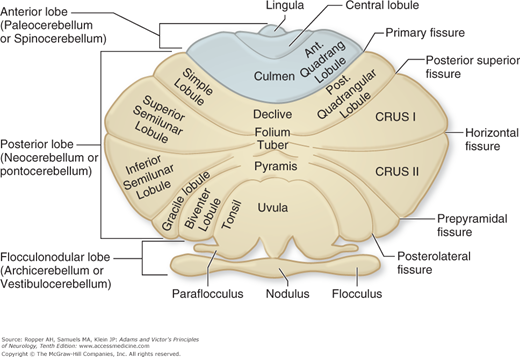
Early studies of the comparative anatomy and fiber connections of the cerebellum led to its subdivision into three parts (Fig. 5-1): (1) The flocculonodular lobe, located inferiorly, which is phylogenetically the oldest portion of the cerebellum and is much the same in all animals (hence archicerebellum). It is separated from the main mass of the cerebellum, or corpus cerebelli, by the posterolateral fissure. (2) The anterior lobe, or paleocerebellum, which is the portion rostral to the primary fissure. In lower animals, the anterior lobe constitutes most of the cerebellum, but in humans it is relatively small, consisting of the anterosuperior vermis and the contiguous paravermian cortex. (3) The posterior lobe, or neocerebellum, consisting of the middle divisions of the vermis and their large lateral extensions. The major portion of the human cerebellar hemispheres falls into this, the largest, subdivision.
This anatomic subdivision corresponds roughly with the distribution of cerebellar function based on the arrangement of its afferent fiber connections. The flocculonodular lobe receives special proprioceptive impulses from the vestibular nuclei and is therefore also referred to as the vestibulocerebellum; it is concerned essentially with equilibrium. The anterior vermis and part of the posterior vermis are referred to as the spinocerebellum, since projections to these parts derive to a large extent from the proprioceptors of muscles and tendons in the limbs and are conveyed to the cerebellum in the dorsal spinocerebellar tract (from the lower limbs) and the ventral spinocerebellar tract (upper limbs). The main influence of the spinocerebellum appears to be on posture and muscle tone. The neocerebellum derives its afferent fibers indirectly from the cerebral cortex via the pontine nuclei and brachium pontis, hence the designation pontocerebellum. This portion of the cerebellum is concerned primarily with the coordination of skilled movements that are initiated at a cerebral cortical level. It is now appreciated that certain portions of the cerebellar hemispheres are also involved to some extent in tactual, visual, auditory, and even visceral functions.
Largely on the basis of ablation experiments in animals, three characteristic physiologic patterns corresponding to these major divisions of the cerebellum have been delineated. These constellations find some similarities in the clinical syndromes that are observed when various parts of the cerebellum are damaged and special terminology is applied to the corresponding clinical findings in patients. Lesions of the nodulus and flocculus have been associated with a disturbance of equilibrium and frequently with nystagmus; individual movements of the limbs are not affected. Anterior lobe ablation in primates results in increased shortening and lengthening reactions, somewhat increased tendon reflexes, and an exaggeration of the postural reflexes, particularly the “positive supporting reflex,” in animals, which consists of extension of the limb in response to light pressure on the foot pad. Ablation of a cerebellar hemisphere in cats and dogs yields inconsistent results, but in monkeys it causes hypotonia and clumsiness of the ipsilateral limbs; if the dentate nucleus is included in the hemispheric ablation, these abnormalities are more enduring and the limbs also show an ataxic, or “intention” tremor.
The studies of Chambers and Sprague and of Jansen and Brodal have demonstrated that in respect to both its afferent and efferent projections, the cerebellum is organized into longitudinal (sagittal) rather than transverse zones. There are three longitudinal zones—the vermian, paravermian or intermediate, and lateral—and there seems to be considerable overlap from one to another. Chambers and Sprague, on the basis of their investigations in cats, concluded that the vermian zone coordinates movements of the eyes and body with respect to gravity and movement of the head in space. The intermediate zone, which receives both peripheral and central projections (from motor cortex), influences postural tone and also individual movements of the ipsilateral limbs. The lateral zone is concerned mainly with coordination of movements of the ipsilateral limbs but is also involved in other functions.
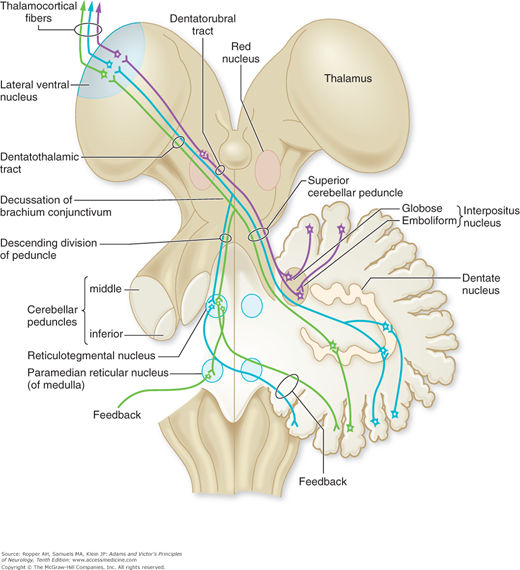
The efferent fibers of the cerebellar cortex, which consist essentially of the axons of Purkinje cells, project onto the deep cerebellar nuclei (see below). The projections from Purkinje cells are inhibitory whereas those from the nuclei are excitatory on other parts of the motor nervous system. According to the scheme of Jansen and Brodal, cells of the vermis project mainly to the fastigial nucleus; those of the intermediate zone, to the globose and emboliform nuclei (that are combined in humans as the interpositus nucleus); and those of the lateral zone, to the dentate nucleus.
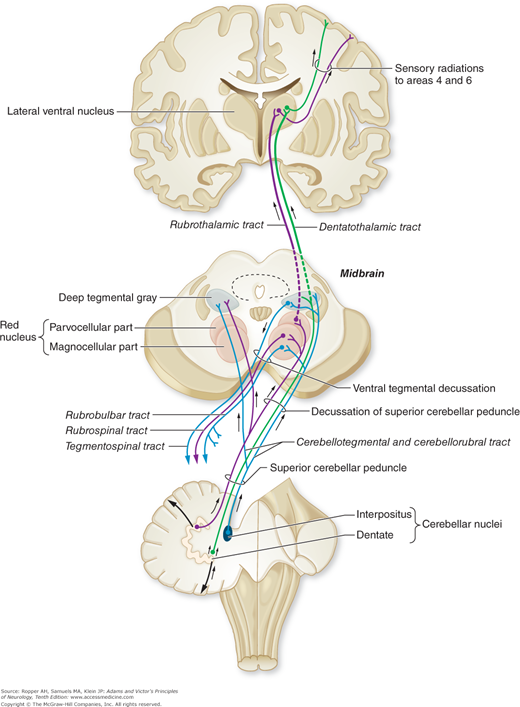
The deep cerebellar nuclei, in turn, project to the cerebral cortex and certain brainstem nuclei via two main pathways: fibers from the dentate, emboliform, and globose nuclei form the superior cerebellar peduncle, enter the upper pontine tegmentum as the brachium conjunctivum, decussate at the level of the inferior colliculus, and ascend to the ventrolateral nucleus of the thalamus and, to a lesser extent, to the intralaminar thalamic nuclei (Fig. 5-2). Some of the ascending fibers, soon after their decussation, synapse in the red nucleus, but most of them traverse this nucleus without terminating, and pass on to the thalamus. Ventral thalamic nuclear groups that receive these ascending efferent fibers project to the supplementary motor cortex of that side. Since the pathway from the cerebellar nuclei to the thalamus and then on to the motor cortex is crossed, and the connection from the motor cortex through the corticospinal is again crossed, the effects of a lesion in one cerebellar hemisphere are manifest by signs on the ipsilateral side of the body.
One special pathway forms a loop, called the Guillain-Mollaret triangle that is of clinical interest. A small group of fibers of the superior cerebellar peduncle, following their decussation, descend in the ventromedial tegmentum of the brainstem via the central tegmental fasciculus and terminate in the reticulotegmental and paramedian reticular nuclei of the pons and inferior olivary nuclei of the medulla. These nuclei, in turn, project via the inferior cerebellar peduncle back to the cerebellum, mainly the anterior lobe, thus completing a cerebellar–reticular–cerebellar feedback system (Fig. 5-3). Several clinical syndromes result from lesions in the loop, notably palatal myoclonus, one of the few disorders of involuntary movement that continues during sleep.
The fastigial nucleus sends fibers to the vestibular nuclei of both sides and, to a lesser extent, to other nuclei of the reticular formation of the pons and medulla. There are also direct fiber connections with the alpha and gamma motor neurons of the spinal cord. The inferior olivary nuclei project via the restiform body (inferior cerebellar peduncle) to the contralateral cerebellar cortex and corresponding parts of the deep cerebellar nuclei. Thus the cerebellum influences motor activity through its connections with the motor cortex and brainstem nuclei and their descending motor pathways. Chapter 4 details the integration of basal ganglionic influences with those of the cerebellum by their confluence in the anterior thalamic nuclei.
Clinicopathologic observations indicate that the cerebellar cortex, and the anterior lobe in particular, is organized somatotopically. This view has been amply confirmed experimentally by mapping of evoked potentials from the cerebellar cortex elicited by a variety of sensory stimuli, and an analysis of the subtle motor effects produced by stimulation of specific parts of the cerebellar cortex. The topographic sensory representation of body parts based on these experimental observations is assumed to be similar to the motor map but the latter is probably not as distinct. The similarity between this scheme and the one derived from the study of human disease becomes apparent when one considers the results of cerebellar lesions discussed further on. Diffuse degenerations of the cerebellum, of course, have widespread effects, including motor, articulatory, gait and eye movements, and subtle behavioral influences.
The physiologic studies of Allen and Tsukahara and of Thach and colleagues have greatly increased our knowledge of the role of the deep cerebellar nuclei. These investigators studied the effects of cooling the deep nuclei during a projected movement in the macaque monkey. Their observations, coupled with established anatomic data, permit the following conclusions. The dentate nucleus receives information from the premotor and supplementary motor cortices via the pontocerebellar system and helps to initiate volitional movements. The latter are accomplished via efferent projections from the dentate nucleus to the ventrolateral thalamus and motor cortex. The dentatal neurons were shown to fire just before the onset of volitional movements, and inactivation of the dentatal neurons delayed the initiation of such movements. The interpositus nucleus also receives cerebrocortical projections via the pontocerebellar system; in addition, it receives spinocerebellar projections via the intermediate zone of the cerebellar cortex. The latter projections convey information from Golgi tendon organs, muscle spindles, cutaneous afferents, and spinal cord interneurons involved in movement. The interpositus nucleus fires in relation to a movement once it has started. Also, the prepositus nucleus appears to be responsible for making volitional oscillations (alternating movements). Its cells fire in tandem with these actions, and their regularity and amplitude are impaired when these cells are inactivated. In addition, Thach has pointed out that the nucleus interpositus normally damps physiologic tremor; he has suggested that this may play a part in the genesis of so-called intention tremor described further on. The fastigial nucleus controls antigravity and other muscle synergies in standing and walking; ablation of this nucleus greatly impairs these motor activities.
Coordinated and fluid movements of the limbs and trunk result from a neuronal organization in the cerebellum that permits an ongoing and almost instantaneous comparison between desired and actual movements while the movements are being carried out. An enormous number of neurons are committed to these tasks, as attested by the fact that the cerebellum contributes only 10 percent to the total weight and volume of the brain but contains half of the brain’s neurons. Also, it has been estimated that there are 40 times more afferent axons than efferent axons in the various cerebellar pathways—a reflection of the enormous amount of incoming (sensory) information that is required for the control of motor function.
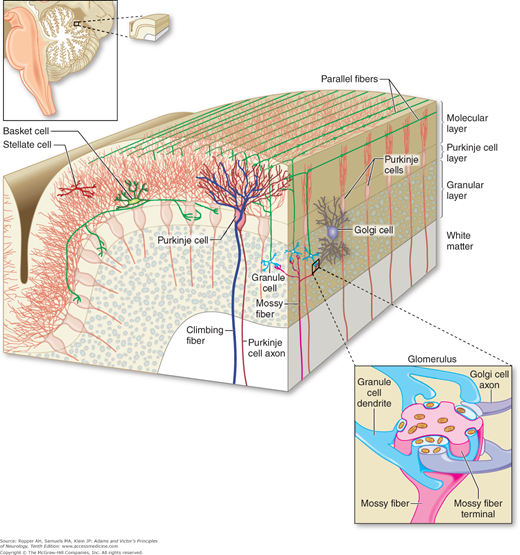
The cerebellar cortex is configured as a stereotyped three-layered structure containing five types of neurons (Fig. 5-4). In its relatively regular geometry, it is similar to the columnar architecture of the cerebral cortex, but it differs in the greater degree of intracortical feedback between neurons and the convergent nature of input fibers. The outermost “molecular” layer of the cerebellum contains two types of inhibitory neurons, the stellate cells and the basket cells. They are interspersed among the dendrites of the Purkinje cells, the cell bodies of which lie in the underlying layer. The Purkinje cell axons constitute the main output of the cerebellum, which is directed at the deep cerebellar and vestibular nuclei described above. Purkinje cells are likewise entirely inhibitory and utilize the neurotransmitter gamma-aminobutyric acid (GABA). The innermost “granular” layer contains an enormous number of densely packed granule cells and a few larger Golgi interneurons. Axons of the granule cells travel long distances as “parallel fibers,” which are oriented along the long axis of the folia and form excitatory synapses with Purkinje cells. Each Purkinje cell is influenced by as many as a million granule cells to produce a single electrical “simple spike.”
Figure 5-4.
Anatomic organization of the cerebellar cortex in a longitudinal and transverse section of a folium. Shown are the relationships between (a) climbing fibers and Purkinje cells, (b) mossy fibers and both granule cells and Golgi cells, and (c) the parallel fibers that course longitudinally and connect these three main cell types. (Reproduced with permission from Kandel ER, Schwartz JH, Jessel TM: Principles of Neural Science, 5th ed. New York, McGraw-Hill, 2013.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree