Pain: Introduction
Pain is an important sign of illness, and it stands preeminent among all the sensory experiences by which humans judge the existence of disease within themselves. Indeed, pain is the most common medical symptom. Relatively few diseases do not have a painful phase and in many, pain is a characteristic without which diagnosis must be in doubt.
The painful experiences pose manifold problems in virtually every field of medicine; physicians must therefore be prepared to recognize disease in patients who have felt only the first rumblings of discomfort, before other symptoms and signs have appeared. Even more problematic are patients who seek treatment for pain that appears to have little or no structural basis; further inquiry may disclose that fear of serious disease, worry, or depression has aggravated some relatively minor ache or that the complaint of pain has become the means of seeking attention, drugs or monetary compensation. They must also cope with the “difficult” pain patients in whom no amount of investigation brings to light either medical or psychiatric illness. Finally, the physician must be prepared to manage patients who require relief from intractable pain caused by established and incurable disease. To deal intelligently with pain problems requires familiarity with the anatomy of sensory pathways and the sensory supply of body segments as well as insight into the psychological factors that influence the perception of and reaction to pain.
The ambiguity with which the term pain is used is responsible for some of our difficulty in understanding it. One aspect, the easier to comprehend, is the transmission of impulses along certain pathways in response to potentially tissue-damaging stimuli, i.e., nociception. Far more abstruse is its quality as a mental state intimately linked to emotion, i.e., the quality of anguish or suffering—”a passion of the soul,” in the words of Aristotle—which defies definition and quantification. This duality (nociception and suffering) is of practical importance for certain drugs or surgical procedures, such as cingulotomy, may reduce the patient’s reaction to painful stimuli, leaving awareness of the sensation largely intact. Alternatively, interruption of certain neural pathways may abolish all sensation in an affected part but the symptom of pain may persist (i.e., denervation dysesthesia or anesthesia dolorosa), even in an amputated limb (“phantom pain”). Finally, unlike most sensory modalities—which are aroused by a specific stimulus such as touch-pressure, heat, or cold—pain can be evoked by any one of these stimuli if it is intense enough.
It is apparent to us that in highly specialized medical centers, and often even in “pain centers,” few physicians are capable of handling difficult and unusual pain problems in any comprehensive way. In fact, it is to the neurologist that other physicians regularly turn for help with these matters. Although much has been learned about the anatomy of pain pathways, their physiologic mechanisms, and which structures to ablate in order to produce analgesia, relatively little is known about which patients should be subjected to these destructive operations or how to manage their pain by medical means. The practice of pain medicine challenges every thoughtful physician, for it demands a high degree of skill in medicine, neurology, and psychiatry.
Anatomy and Physiology of Pain
For more than a century, views on the nature of pain sensation have been dominated by two major theories. One, the specificity theory, was from the beginning associated with the name of von Frey. He asserted that the skin consisted of a mosaic of discrete sensory spots and that each spot, when stimulated, gave rise to one sensation—either pain, pressure, warmth, or cold; in his view, each of these sensations had a distinctive end organ in the skin and each stimulus-specific end organ was connected by its own private pathway to the brain. A second theory was that of Goldscheider, who abandoned his own earlier discovery of pain spots to argue that they simply represented pressure spots, a sufficiently intense stimulation of which could produce pain. According to the latter theory, there were no distinctive pain receptors, and the sensation of pain was the result of the summation of impulses excited by pressure or thermal stimuli applied to the skin. Originally called the intensivity theory, it later became known as the pattern or summation theory.
In an effort to conciliate the pattern and specificity theories, Head and colleagues, in 1905, formulated a novel concept of pain sensation, based on observations that followed his purposeful division of the cutaneous branch of the radial nerve in his own forearm. The zone of impaired sensation contained an innermost area in which superficial sensation was completely abolished. This was surrounded by a narrower (“intermediate”) zone, in which pain sensation was preserved but poorly localized; extreme degrees of temperature were recognized in the intermediate zone but perception of touch, lesser differences of temperature, and two-point discrimination were abolished. To explain these findings, Head postulated the existence of two systems of cutaneous receptors and conducting fibers: (1) an ancient protopathic system, subserving pain and extreme differences in temperature and yielding ungraded, diffuse impressions of an all-or-none type; and (2) a more recently evolved epicritic system, which mediated touch, two-point discrimination, and lesser differences in temperature, as well as localized pain. The pain and hyperesthesia that follow damage to a peripheral nerve were attributed to a loss of inhibition that was normally exerted by the epicritic upon the protopathic system. This theory was used for many years to explain the sensory alterations that occur with both peripheral and central (thalamic) lesions. It lost credibility for several reasons but mainly because Head’s original observations and deductions upon which they were based could not be confirmed (see Trotter and Davies; also Walshe). Nevertheless, both fast and slow forms of pain conduction were later corroborated (see below).
A later refinement of the pattern and specificity concepts of pain was made in 1965 when Melzack and Wall articulated their “gate-control” theory. They observed, in decerebrate and spinal cats, that peripheral stimulation of large myelinated fibers produced a negative dorsal root potential and that stimulation of small unmyelinated C (pain) fibers caused a positive dorsal root potential. They postulated that these potentials, which were a reflection of presynaptic inhibition or excitation, modulated the activity of secondary transmitting neurons (T cells) in the dorsal horn and that this modulation was mediated through inhibitory (I) cells. The essence of this theory was that the large-diameter fibers excited the I cells, which, in turn, caused a presynaptic inhibition of the T cells; conversely, the small pain afferents inhibited the I cells, leaving the T cells in an excitatory state. Melzack and Wall emphasized that pain impulses from the dorsal horn must also be under the control of a descending system of fibers from the brainstem, thalamus, and limbic lobes.
At first the gate-control mechanisms seemed to offer an explanation of the pain of ruptured disc and of certain chronic neuropathies (particularly those with large fiber out-fall) and attempts were made to relieve pain by subjecting the peripheral nerves and dorsal columns (presumably their large myelinated fibers) to sustained transcutaneous electrical stimulation. Such selective stimulation would theoretically “close” the gate. In some clinical situations these procedures have indeed given relief from pain, but not necessarily as a result of stimulation of large myelinated fibers alone (see Taub and Campbell). But in a number of other instances relating to pain in large- and small-fiber neuropathies, the clinical behavior has been quite out of keeping with what one would expect on the basis of the gate-control mechanism. As with preceding theories, flaws have been exposed in the physiologic observations on which the theory is based. These and other aspects of the gate-control theory of pain have been critically reviewed by Nathan.
During the last few decades there has been a significant accrual of information on cutaneous sensibility, demanding modification of earlier anatomic–physiologic and clinical concepts. Interestingly, much of this information is still best described and rationalized in the general framework of the oldest theory, that of specificity, as is evident from the ensuing discussion on pain and that on other forms of cutaneous sensibility in the next chapter.
In terms of peripheral pain mechanisms, there is indeed a high degree, though not absolute, specificity in the von Frey sense. In keeping with distinctions between nerve types, the sensory (and motor) fibers have been classified according to their size and function (Table 8-1). It is now well established that two types of afferent fibers in the distal axons of primary sensory neurons respond maximally to nociceptive (i.e., potentially tissue-damaging) stimuli. One type is the very fine, unmyelinated, slowly conducting C fiber (0.3 to 1.1μ in diameter); the other is the thinly myelinated, more rapidly conducting A-δ fiber (2 to 5μ in diameter). The peripheral terminations of both of these primary pain afferents or receptors are the free, profusely branched nerve endings in the skin and other organs; these are covered by Schwann cells but contain little or no myelin.
FIBER TYPE | ALTERNATIVE DESIGNATION | FIBER DIAMETER | CONDUCTION VELOCITY (m/s) | FUNCTION AND SYMPTOMS OF DYSFUNCTION |
|---|---|---|---|---|
A-α and -β | II | 5–20 | 30–70 | Touch, pressure |
Large, heavily myelinated | ||||
A-γ | Ia | 3–6 | 15–30 | Spindle afferents |
A-δ | III | 2–5 | 12–30 | Pain and temperature, soma touch (sharp, lancinating, prickly pain) |
Small, thinly myelinated | ||||
B | 1–3 | 3–15 | ||
C | IV | 0.3–1.1 | 0.5–2 | Slow pain and temperature (dull, burning, poorly localized pain) |
Small, unmyelinated; polymodal |
There is considerable evidence, based on their response characteristics, that a degree of subspecialization exists within these freely branching, nonencapsulated endings and their small-fiber afferents. Three categories of free endings or receptors are recognized: mechanoreceptors, thermoreceptors, and polymodal nociceptors. Each ending transduces stimulus energy into an action potential in the distal nerve membranes. The first two types of receptors are activated by innocuous mechanical and thermal stimulation, respectively; the mechanoeffects are transmitted by both A-δ and C fibers and the thermal effects mostly by C fibers. The majority of C fibers are polymodal and are most effectively excited by noxious or tissue-damaging stimuli, but they can respond to both mechanical and thermal stimuli and to chemical mediators such as those associated with inflammation. Moreover, certain A-δ fibers respond to light touch, temperature, and pressure as well as to pain stimuli and are capable of discharging in proportion to the intensity of the stimulus. The stimulation of single fibers by intraneural electrodes indicates that they can also convey information concerning the nature and location of the stimulus. These observations on the polymodal functions of A-δ and C fibers would explain the earlier observations of Lele and Weddell that modes of sensation other than pain can be evoked from structures such as the cornea, which is innervated solely by free nerve endings.
The manner in which painful stimuli are translated into electrical depolarizations in nerve endings is beginning to be understood. A number of specialized molecules, when activated by noxious stimuli, open cationic channels in membranes of the nerve ending. Opening of these channels, in turn, activates voltage-gated sodium channels and generates an action potential in the sensory axon. Mannion and Woolf have summarized the regulation and activation of these receptor molecules.
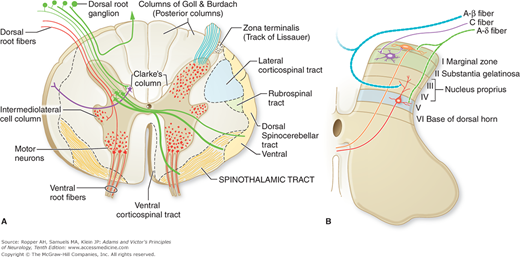
The peripheral afferent pain fibers of both A-δ and C types have their cell bodies in the dorsal root ganglia; central extensions of these nerve cells project, via the dorsal root, to the dorsal horn of the spinal cord (or, in the case of cranial pain afferents, to the spinal trigeminal nucleus, the medullary analogue of the dorsal horn). The pain afferents occupy mainly the lateral part of the root entry zone. Within the spinal cord, many of the thinnest fibers (C fibers) form a discrete bundle, the tract of Lissauer (Fig. 8-1A). That the tract of Lissauer is predominantly a pain pathway is shown (in animals) by the ipsilateral segmental analgesia that results from its transection but it contains deep sensory or propriospinal fibers as well. Although it is customary to speak of a lateral and medial division of the posterior root (the former contains small pain fibers and the latter, large myelinated fibers), the separation into discrete functional bundles is not complete, and in humans the two groups of fibers cannot be differentially interrupted by selective rhizotomy.
Figure 8-1.
A. Spinal cord in transverse section illustrating the course of the afferent fibers and the major ascending pathways. Fast-conducting pain fibers are not confined to the spinothalamic tract but are scattered diffusely in the anterolateral funiculus (see also Fig. 8-3). (Adapted from Martin JH: Neuroanatomy: Text and Atlas. New York, McGraw-Hill, 2003, with permission.) B. Transverse section through a cervical segment of the spinal cord illustrating the subdivision of the gray matter into laminae according to Rexed and the entry and termination of the main sensory fibers. (Adapted from Fields HL: Pain. New York, McGraw-Hill, 1987, with permission.)
(See Fig. 9.1) Each sensory unit (the sensory nerve cell in the dorsal root ganglion, its central and peripheral extensions, and cutaneous and visceral endings) has a unique topography that is maintained throughout the sensory system from the periphery to the sensory cortex. The discrete segmental distribution of the sensory units permits the construction of sensory maps, so useful to clinicians (see Fig. 9-1). This aspect of sensory anatomy is elaborated in the next chapter, which includes maps of the sensory dermatomes and cutaneous nerves. However, as a means of quick orientation to the topography of peripheral pain pathways, it is useful to remember that the facial structures and anterior cranium lie in the fields of the trigeminal nerves; the back of the head, second cervical; the neck, third cervical; the epaulet area, fourth cervical; the deltoid area, fifth cervical; the radial forearm and thumb, sixth cervical; the index and middle fingers, seventh cervical; the little finger and ulnar border of hand and forearm, eighth cervical–first thoracic; the nipple, fifth thoracic; the umbilicus, tenth thoracic; the groin, first lumbar; the medial side of the knee, third lumbar; the great toe, fifth lumbar; the little toe, first sacral; the back of the thigh, second sacral; and the genitoanal zones, third, fourth, and fifth sacral segments. The distribution of pain fibers from deep structures, although not fully corresponding to those from the skin, also follows a segmental pattern. The first to fourth thoracic nerve roots are the important sensory pathways for the heart and lungs; the sixth to eighth thoracic, for the upper abdominal organs; and the lower thoracic and upper lumbar, for the lower abdominal viscera. These areas of projection from visceral structures roughly correspond to the areas of adjacent root innervation, with some exceptions because of routing of sensory nerves to organs that migrate with development.
Neurologically relevant maps of pain projection from the bones, ligaments, and adjacent musculoskeletal structures have been termed sclerotomes; they differ slightly in their distribution from the dermatomes. A further discussion of referred pain and a figure comparing sclerotomes and dermatomes is given later in the chapter.
The afferent pain fibers, after traversing the tract of Lissauer, terminate in the posterior gray matter or dorsal horn, predominantly in the marginal zone. Most of the fibers terminate within the segment of their entry into the cord; some extend ipsilaterally to one or two adjacent rostral and caudal segments; and some project, via the anterior commissure, to the contralateral dorsal horn. The cytoarchitectonic studies of Rexed in the cat (the same organization pertains in primates and probably in humans) have shown that second-order neurons, the sites of synapse of afferent sensory fibers in the dorsal horn, are arranged in a series of six layers or laminae (Fig. 8-1B). Thinly myelinated (A-δ) fibers terminate principally in lamina I of Rexed (marginal cell layer of Waldeyer) and also in the outermost part of lamina II; some A-δ pain fibers penetrate the dorsal gray matter and terminate in the lateral part of lamina V. Unmyelinated (C) fibers terminate in lamina II (substantia gelatinosa). Yet other cells that respond to painful cutaneous stimulation are located in ventral horn laminae VII and VIII. The latter neurons are responsive to descending impulses from brainstem nuclei as well as segmental sensory impulses. From these cells of termination, second-order axons connect with ventral and lateral horn cells in the same and adjacent spinal segments and subserve both somatic and autonomic reflexes. The main bundle of secondary neurons subserving pain sensation projects contralaterally (and to a lesser extent ipsilaterally) to higher levels; this constitutes the spinothalamic tract, discussed below.
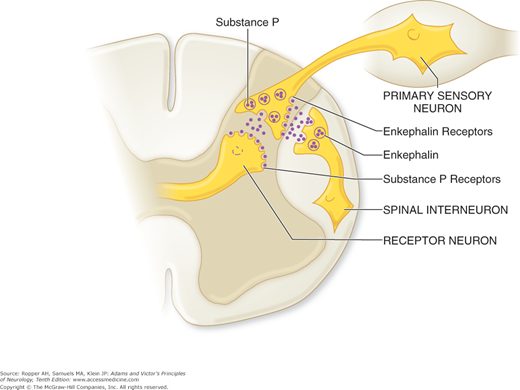
A number of important observations have been made concerning the mode of transmission and modulation of pain impulses in the dorsal horn and brainstem. Excitatory amino acids (glutamate, aspartate) and nucleotides such as adenosine triphosphate (ATP) are the putative transmitters at terminals of primary A-δ sensory afferents. Also, A-δ pain afferents, when stimulated, release several neuromodulators that play a role in the transmission of pain sensation. Slower neurotransmission by C neurons involves other substances, of which the most important is the 11-amino-acid peptide known as substance P (“P” for powder extracted from animal tissue and urine by von Euler in 1931). In animals, substance P excites nociceptive dorsal root ganglion and dorsal horn neurons; furthermore, destruction of substance P fibers produces analgesia. In patients with the rare condition of congenital neuropathy and insensitivity to pain, there is a marked depletion of dorsal horn substance P.
A large body of evidence indicates that opiates are important modulators of pain impulses as they are relayed through the dorsal horn and through nuclei in the medulla and midbrain. Thus, opiates have been noted to decrease substance P; at the same time, flexor spinal reflexes, which are evoked by segmental pain, are reduced. Opiate receptors of three types are found on both presynaptic primary afferent terminals and postsynaptic dendrites of small neurons in lamina II. Moreover, lamina II neurons, when activated, release enkephalins, endorphins, and dynorphins—all of which are endogenous, morphine-like peptides that bind specifically to opiate receptors and inhibit pain transmission at the dorsal horn level. The subject of pain modulation by opiates and endogenous morphine-like substances is elaborated further on.
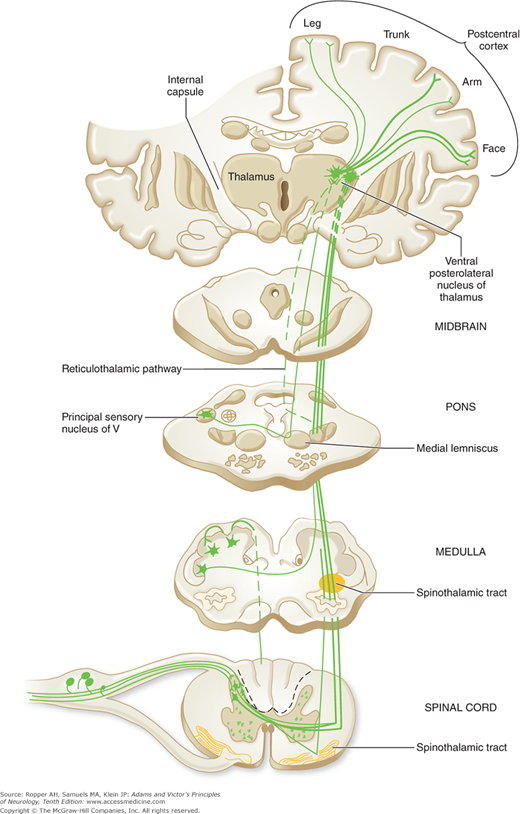
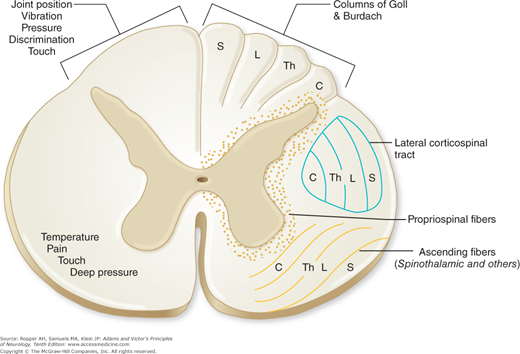
As indicated above, axons of secondary neurons that sub-serve pain sensation originate in laminae I, II, V, VII, and VIII of the spinal gray matter. The principal bundle of these axons decussates in the anterior spinal commissure and ascends in the anterolateral fasciculus of the opposite side of the cord as the spinothalamic tract to terminate in brainstem and thalamic structures (Fig. 8-2). It is of clinical consequence that the axons carrying pain impulses from each dermatome decussate one to three segments rostral to the level of root entry. For this reason, a discrete lesion of the lateral spinal cord creates a loss of pain and thermal sensation of the contralateral trunk, the dermatomal level of which is two to three segments below that of the spinal cord lesion. As the ascending fibers cross the cord, they are added to the inner side of the spinothalamic tract (the principal afferent pathway of the anterolateral fasciculus), so that the longest fibers from the sacral segments come to lie most superficially and fibers from successively more rostral levels occupy progressively deeper positions (Fig. 8-3). This somatotopic arrangement is of importance to the neurosurgeon performing an operation for pain relief, insofar as the depth to which the funiculus is cut will govern the level of analgesia that is achieved; for the neurologist, it provides an explanation of the pattern of “sacral sparing” of pain and thermal sensation created by centrally placed lesions of the spinal cord. The termination of this tract, mainly in the thalamus, is described further on.
Figure 8-2.
The spinothalamic tract (pain, thermal sense) is shown. In the bottom section, the fibers that form the spinothalamic tract cross over two or three segment rostral to their entry into the cord, not at the same level as depicted. Offshoots from the ascending anterolateral fasciculus (spinothalamic tract) to nuclei in the medulla, pons, and mesencephalon and nuclear terminations of the tract are indicated. The cortical representation of sensation is shown grossly; it is shown more explicitly in Fig. 9-5 and discussed in Chap. 9. The lemniscal (posterior column) system is shown in Fig. 9-4.
Figure 8-3.
Spinal cord showing the segmental and laminated arrangement of nerve fibers within major tracts. On the left side are indicated the “sensory modalities” that appear to be mediated by the two main ascending pathways. C, cervical; L, lumbar; S, sacral; Th, thoracic. (Adapted by permission from Brodal A: Neurological Anatomy, 3rd ed. New York, Oxford University Press, 1981.)
In addition to the anterolateral spinothalamic tract—a fast-conducting pathway that projects directly to the thalamus—the anterolateral fasciculus of the cord contains several more slowly conducting, medially placed systems of fibers. One such group of fibers projects directly to the reticular core of the medulla and midbrain and then to the medial and intralaminar nuclei of the thalamus; these fibers are referred to as the spinoreticulothalamic or paleospinothalamic pathway. At the level of the medulla, these fibers synapse in the nucleus gigantocellularis; more rostrally, they connect with nuclei of the parabrachial region, midbrain reticular formation, periaqueductal gray matter, and hypothalamus. A second, more medially placed pathway in the anterolateral cord ascends to the brainstem reticular core via a series of short interneuronal links. It is not clear whether these spinoreticular fibers are collaterals of the spinothalamic tracts, as Cajal originally stated, or whether they represent an independent system, as more recent data seem to indicate. Probably both statements are correct. There is also a third, direct spinohypothalamic pathway in the anterolateral fasciculus.
The conduction of diffuse, poorly localized pain arising from deep and visceral structures (gut, periosteum, peritoneum) has been ascribed to these slow-conducting, indirect pathways. Melzack and Casey have proposed that this fiber system (which they refer to as paramedian), with its diffuse projection via brainstem and thalamus to the limbic and frontal lobes, subserves the affective aspects of pain, i.e., the unpleasant feelings engendered by pain. It is evident that these spinoreticulothalamic pathways continue to evoke the psychic experience of pain even when the direct spinothalamic pathways have been interrupted. However, it is the direct spinothalamic pathway, which projects to the ventroposterolateral (VPL) nucleus of the thalamus and thence to discrete areas of the sensory cortex, that subserves the sensory-discriminative aspects of pain, i.e., the processes that underlie the localization, quality, and possibly the intensity of the noxious stimulus. Also, the pathways for visceral pain from the esophagus, stomach, small bowel, and proximal colon are carried largely in the vagus nerve and terminate in the nucleus of the solitary tract (nucleus tractus solitarius [NTS]) before projecting to the thalamus, as described below. Other abdominal viscera still activate the NTS when the vagus is severed in animals, probably transmitting impulses through the splanchnic plexus.
It should be emphasized that the foregoing data concerning the cells of termination of cutaneous nociceptive stimuli and the cells of origin of ascending spinal afferent pathways have all been obtained from studies in animals (including monkeys). In humans, the specific cells of origin of the direct spinothalamic tract fibers have not been fully identified. Information about this pathway in humans has been derived from the study of postmortem material and from the examination of patients subjected to anterolateral cordotomy for intractable pain. What can be stated of clinical importance is that unilateral section of the anterolateral funiculus produces a relatively complete loss of pain and thermal sense on the opposite side of the body, extending to a level two or three segments below the lesion as noted earlier. After a variable period of time, pain sensibility usually returns, probably being conducted by pathways that lie outside the anterolateral quadrants of the spinal cord that gradually increase their capacity to conduct pain impulses. One of these is a longitudinal polysynaptic bundle of small myelinated fibers in the center of the dorsal horn (the dorsal intracornual tract); another consists of axons of lamina I cells that travel in the dorsal part of the lateral funiculus.
The direct spinothalamic fibers separate into two bundles as they approach the thalamus. The lateral division terminates in the ventrobasal and posterior groups of nuclei, the most important of which is the VPL nucleus. The medial contingent terminates mainly in the intralaminar complex of nuclei and in the nucleus submedius. Spinoreticulothalamic (paleospinothalamic) fibers project onto the medial intralaminar (primarily parafascicular and centrolateral) thalamic nuclei; i.e., they overlap with the terminations of the medially projecting direct spinothalamic pathway. Projections from the dorsal column nuclei, which have a modulating influence on pain transmission, are mainly to the ventrobasal and ventroposterior group of nuclei. Each of the four thalamic nuclear groups that receives nociceptive projections from the spinal cord has a distinct cortical projection and each is thought to play a different role in pain sensation (see below).
One practical conclusion to be reached from these anatomic and physiologic studies is that, at thalamic levels, fibers and cell stations transmitting the nociceptive impulses are not organized into discrete loci. In general, neurophysiologic evidence indicates that as one ascends from peripheral nerve to spinal, medullary, mesencephalic, thalamic, and limbic levels, the predictability of neuron responsivity to noxious stimuli diminishes. Thus it comes as no surprise that neurosurgical lesions that interrupt afferent pathways at progressively higher levels of the brainstem and thalamus become decreasingly successful.
The ventrobasal thalamic complex and the ventroposterior group of nuclei project to two main cortical areas: the primary sensory (postcentral) cortex (a small number terminate in the precentral cortex) and the upper bank of the sylvian fissure. These cortical projections are described more fully in Chap. 9 but it can be stated here that they are concerned mainly with the reception of tactile and proprioceptive stimuli and with all discriminative sensory functions, including pain. The extent to which either cortical area is activated by thermal and painful stimuli is uncertain. Certainly, stimulation of these (or any other) cortical areas in a normal, alert human being does not produce pain. The intralaminar nuclei, which also project to the hypothalamus, amygdaloid nuclei, and limbic cortex, probably mediate the arousal and affective aspects of pain and autonomic responses.
The thalamic projection to the primary sensory cortex that is distributed mainly along the postcentral gyrus of the anterior parietal lobe is shown in Fig. 9-5 (the “sensory homunculus”). The cortical representation allows for accurate localization of the site of origin of a painful stimulus but the notion that thalamic projections terminate solely in this region is an oversimplification.
Thalamic and cerebral cortical localization of visceral sensation is not well known. However, cerebral evoked potentials and increased cerebral blood flow (by positron emission tomography [PET] studies) have been demonstrated in the thalamus and pre- and postcentral gyri of patients undergoing rectal balloon distention (Silverman et al; Rothstein et al).
The discovery of a system of descending fibers and way stations that modulate activity in nociceptive pathways has proved to be a major addition to our knowledge of pain. The endogenous pain control system that has been studied most extensively emanates from the frontal cortex and hypothalamus and projects to cells in the periaqueductal region of the midbrain and then passes to the ventromedial medulla. From there it descends in the dorsal part of the lateral fasciculus of the spinal cord to the posterior horns (laminae I, II, and V; see further discussion under “Endogenous Pain-Control Mechanisms”). Several other descending pathways, noradrenergic and serotonergic, arise in the locus ceruleus, dorsal raphe nucleus, and nucleus reticularis gigantocellularis and are also important modifiers of the nociceptive response. The clinical significance of these pain-modulating pathways, still under study, is discussed further on.
Physiologic Aspects of Pain
The stimuli that activate pain receptors vary from one tissue to another. The adequate stimulus for skin is one that has the potential to injure tissue, i.e., pricking, cutting, crushing, burning, and freezing. These stimuli are ineffective when applied to the stomach and intestine, where pain is produced by an engorged or inflamed mucosa, distention or spasm of smooth muscle, and traction on the mesenteric attachment. In skeletal muscle, pain is caused by ischemia (the basis of intermittent claudication), necrosis, hemorrhage, and injection of irritating solutions as well as by injuries of connective tissue sheaths. Prolonged contraction of skeletal muscle evokes an aching type of pain. Ischemia is also the most important cause of pain in cardiac muscle. Joints are insensitive to pricking, cutting, and cautery, but pain can be produced in the synovial membrane by inflammation and by exposure to hypertonic saline. The stretching and tearing of ligaments around a joint can evoke severe pain. Injuries to the periosteum give rise to pain but probably not to other sensations. Blood vessels are a source of pain when pierced by a needle or involved in an inflammatory process. Distention of arteries or veins, as occurs with thrombotic or embolic occlusion, may be sources of pain; other mechanisms of headache relate to traction on arteries or inflammation of the meningeal structures by which they are supported. (The subject of headache and its origins is taken up in Chap. 10.) Pain from intraneural lesions probably arises from the sheaths of the nerves. Nerve root(s) and sensory ganglia, when compressed (e.g., by a ruptured disc), give rise to pain.
With damage to tissue, there is a release of proteolytic enzymes, which act locally on tissue proteins to liberate substances that excite peripheral nociceptors. These pain-producing substances—which include histamine, prostaglandins, serotonin, and similar polypeptides, as well as potassium ions—elicit pain when they are injected intraarterially or applied to the base of a blister. Other pain-producing substances such as kinins are released from sensory nerve endings or are carried there by the circulation. Local vascular permeability is also increased by these substances.
In addition, direct stimulation of nociceptors releases polypeptide mediators that enhance pain perception. The best studied of these is substance P, which is released from the nerve endings of C fibers in the skin during peripheral nerve stimulation. It causes erythema by dilating cutaneous vessels and edema by releasing histamine from mast cells; it also acts as a chemoattractant for leukocytes. This reaction, called neurogenic inflammation by White and Helme, is mediated by antidromic action potentials from the small nerve cells in the spinal ganglia and is the basis of the axon reflex of Lewis; the reflex is abolished in peripheral nerve diseases and can be studied electrophysiologically as an aid to clinical localization.
The threshold for perception of pain, i.e., the lowest intensity of a stimulus recognized as pain, is approximately the same in all persons. Inflammation lowers the threshold for perception of pain by a process called sensitization. This process, termed allodynia, allows ordinarily innocuous stimuli to produce pain in sensitized tissues. The pain threshold is, of course, raised by local anesthetics and by certain lesions of the nervous system as well as by centrally acting analgesic drugs. Mechanisms other than lowering or raising the pain threshold are important as well. Placebos reduce pain in about one-third of the groups of patients in which such effects have been recorded. Acupuncture at sites anatomically remote from painful operative fields also reduces the pain in some individuals. Distraction and suggestion, by turning attention away from the painful part, reduce the awareness of and response to pain but not the threshold for its perception. Strong emotion (fear or rage) suppresses pain, presumably by activation of the above-described descending noradrenergic system. The experience of pain appears to be lessened in manic states and enhanced in depression. Anxious patients in general have the same pain threshold as normal subjects but their reaction may be excessive or abnormal. The pain thresholds of frontal lobotomized subjects are also unchanged but they react to painful stimuli only briefly or casually if at all.
The conscious awareness or perception of pain occurs only when pain impulses reach the thalamocortical level. The precise roles of the thalamus and cortical sensory areas in this mental process are not fully understood. It was believed that the recognition of a noxious stimulus as such is a function of the thalamus and that the parietal cortex is necessary for appreciation of the intensity, localization, and other discriminatory aspects of sensation. This traditional separation of sensation (in this instance, awareness of pain) and perception (awareness of the nature of the painful stimulus) has evolved to the view that sensation, perception, and the various conscious and unconscious responses to a pain stimulus comprise an indivisible process. That the cerebral cortex governs the patient’s reaction to pain cannot be doubted. It is also likely that the cortex can suppress or otherwise modify the perception of pain in the same way that corticofugal projections from the sensory cortex modify the rostral transmission of other sensory impulses from thalamic and dorsal column nuclei. It has been shown that central transmission in the spinothalamic tract can be inhibited by stimulation of the sensorimotor areas of the cerebral cortex, and, as indicated above, a number of descending fiber systems have been traced to the dorsal horn laminae from which this tract originates.
The functional imaging studies by Wager and coworkers has given insights into the ensemble of brain regions that are activated by painful stimuli. In addition to the expected thalamic and parietal sensory regions, the hypothalamus, and both insular and cingulate cortices, are prominently involved, in proportion to the intensity of the stimulus. These investigators have sought to develop an imaging “pain signature” that could, in the future, objectify the pain response. Moreover, physical pain in their experiments could be differentiated from social and emotional pain. Whether this reductionist approach to pain will find clinical use is discussed by Jaillard and Ropper.
An important contribution to our understanding of pain has been the discovery of a neuronal analgesia system that can be activated by the administration of opiates or by naturally occurring brain substances that share the properties of opiates. This endogenous system was first demonstrated by Reynolds, who found that stimulation of the ventrolateral periaqueductal gray matter in the rat produced a profound analgesia without altering behavior or motor activity. Subsequently, stimulation of other discrete sites in the medial and caudal regions of the diencephalon and rostral bulbar nuclei (notably raphe magnus and paragigantocellularis) was shown to have the same effect. Under the influence of such electrical stimulation, the animal could be operated on without anesthesia and move around in an undisturbed manner despite the administration of noxious stimuli. Investigation disclosed that the effect of stimulation-produced analgesia (SPA) is inhibition of the neurons of laminae I, II, and V of the dorsal horn, i.e., the neurons that are activated by noxious stimuli. In human subjects, stimulation of the midbrain periaqueductal gray matter through stereotactically implanted electrodes has also produced a state of analgesia, though not consistently. Other sites in which electrical stimulation is effective in suppressing nociceptive responses are the rostroventral medulla (nucleus raphe magnus and adjacent reticular formation) and the dorsolateral pontine tegmentum. These effects are relayed to the dorsal horn gray matter via a pathway in the dorsolateral funiculus of the spinal cord. Ascending pathways from the dorsal horn, conveying noxious somatic impulses, are also important in activating the modulatory network.
Opiates also act pre- and postsynaptically on the neurons of laminae I and V of the dorsal horn, suppressing afferent pain impulses from both the A-δ and C fibers. Furthermore, these effects can be reversed by the opioid antagonist naloxone. Interestingly, naloxone can reduce some forms of stimulation-produced analgesia. Levine and colleagues have demonstrated that not only does naloxone enhance clinical pain, but it also interferes with the pain relief produced by placebos. These observations suggest that the heretofore mysterious beneficial effects of placebos (and perhaps of acupuncture) may be a result of activation of an endogenous system that shuts off pain through the release of pain-relieving endogenous opioids, or endorphins (see below). Prolonged pain and fear are the most powerful activators of this endogenous opioid-mediated modulating system. The same system is probably operative under a variety of other stressful conditions; for example, some soldiers, wounded in battle, require little or no analgesic medication (“stress-induced analgesia”). The opiates also act at several loci in the brainstem, at sites corresponding with those producing analgesia when stimulated electrically and generally conforming to areas in which neurons with endorphin receptors are localized.
Soon after the discovery of specific opiate receptors in the central nervous system (CNS), several naturally occurring peptides, which proved to have a potent analgesic effect and to bind specifically to opiate receptors, were identified. These endogenous, morphine-like compounds are generically referred to as endorphins, meaning “the morphines within.” The most widely studied are β-endorphin, a peptide sequence of the pituitary hormone β-lipotropin, and two other peptides, enkephalin and dynorphin. They are found in greatest concentration in relation to opiate receptors in the midbrain. At the level of the spinal cord, exclusively enkephalin receptors are found. Figure 8-4 illustrates a theoretical construct of the roles of enkephalin (and substance P) at the point of entry of pain fibers into the spinal cord. A subgroup of dorsal horn interneurons that are in contact with spinothalamic tract neurons also contains enkephalin.