Other Somatic Sensation: Introduction
Sensory and motor functions are interdependent, as was dramatically illustrated by the early animal experiments of Claude Bernard and Charles Sherrington, in which practically all effective movement of a limb was abolished by eliminating only its sensory innervation (sectioning of posterior roots). Interruption of other sensory pathways and destruction of the parietal cortex also has profound effects on motility. To a large extent, human motor activity depends on a constant influx of sensory impulses (most of them not consciously perceived). Sensory motor integration is therefore necessary for normal nervous system function but disease may affect motor or sensory functions independently. There may be loss or impairment of sensory function, and this can represent the principal manifestation of neurologic disease.
This chapter deals with general somatic sensation, i.e., afferent impulses that arise in the skin, muscles, or joints. One form of somatic sensation—pain—was discussed in Chap. 8. Because of its overriding clinical importance, pain has been accorded a chapter of its own, but that chapter and this chapter are of one piece. The special senses—vision, hearing, taste, and smell—are considered in the next section (Chaps. 12, 13, 14, and 15), and visceral sensation, most of which does not reach consciousness, is considered with the disorders of the autonomic nervous system (Chap. 26).
Anatomic and Physiologic Considerations
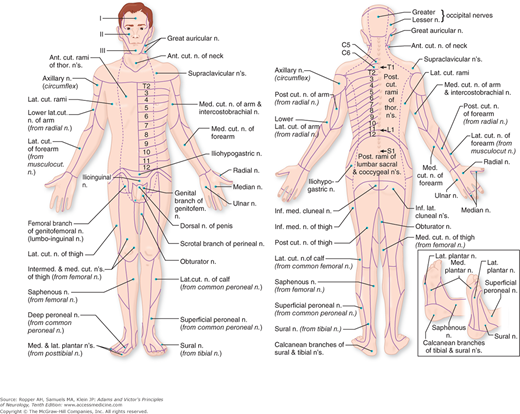
An understanding of sensory disorders depends upon knowledge of functional anatomy. It is necessary to be familiar with the sensory receptors in the skin and deeper structures, the distribution of the peripheral nerves and roots, and the pathways by which sensory impulses are conveyed from the periphery and through the spinal cord and brainstem to the thalamus and cerebral cortex. These aspects of sensory anatomy and physiology were touched upon in Chap. 8 in relation to the perception of pain and are elaborated upon here to include all forms of somatic sensation. An appropriate starting point is Fig. 9-1 that shows the cutaneous distribution of the peripheral nerves.
All sensation depends on impulses that are excited by stimulation of receptors and conveyed to the central nervous system by afferent (sensory) fibers. Sensory receptors are of two general types: those in the skin, mediating superficial sensation (exteroceptors), and those in the deeper somatic structures (proprioceptors). Skin receptors are particularly numerous and transduce four types of sensory experience: warmth, cold, touch, and pain; these are conventionally referred to as sensations or senses, e.g., tactile sensation or sense of touch. Proprioceptors inform us of the position of our body or parts of our body; of the force, direction, and range of movement of the joints (kinesthetic sense); and a sense of pressure, both painful and painless. Histologically, a wide variety of sensory receptors have been described, varying from simple, free dendrite terminals to highly branched and encapsulated structures, the latter bearing the names of the anatomists who first described them (see below). These are called “dendrites” because the direction of flow of physiologic activity and of sensory information from these structures in the periphery is toward the cell body.
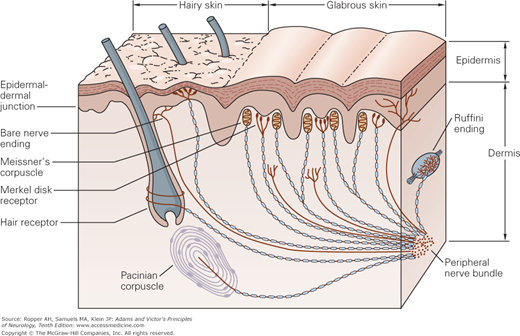
As indicated in the preceding chapter, it had been thought that each of the primary modalities of cutaneous sensation is subserved by a morphologically distinct end organ, each with its separate peripheral nerve fibers. According to this formulation, postulated by von Frey, each type of end organ was thought to respond only to a particular type of stimulus and to subserve a specific modality of sensation: Meissner corpuscles (named after Georg Meissner), touch; Merkel discs (named after Friedrich Sigmund Merkel), pressure; Ruffini plumes (names after Angelo Ruffini), warmth and skin stretch; Krause end bulbs (named after Wilhelm Krause), cold; Pacini (pacinian) corpuscles (named after Filippo Pacini), vibration and tickle; and for pain, nerve endings that not associated with transducer (“free nerve endings”). The last of these are also termed “naked” because they are surrounded by Schwann cells but are not myelinated (Fig. 9-2).
Figure 9-2.
The location and morphology of mechanoreceptors in hairy and hairless (glabrous) skin of the human hand. Receptors are located in the superficial skin, at the junction of the dermis and epidermis, and more deeply in the dermis and subcutaneous tissue. The receptors of the glabrous skin are Meissner’s corpuscles, located in the dermal papillae; Merkel disk receptors, located between the dermal papillae; and bare nerve endings. The receptors of the hairy skin are hair receptors, Merkel’s receptors (having a slightly different organization than their counterparts in the glabrous skin), and bare nerve endings. Subcutaneous receptors, beneath both glabrous and hairy skin, include Pacinian corpuscles and Ruffini endings. Nerve fibers that terminate in the superficial layers of the skin are branched at their distal terminals, innervating several nearby receptor organs; nerve fibers in the subcutaneous layer innervate only a single receptor organ. The structure of the receptor organ determines its physiological function. (Reproduced with permission from Kandel ER, Schwartz JH, Jessell TM: Principles of Neural Science, 4th ed. New York: McGraw-Hill, 2000.)
This specificity theory, as it came to be called, has held up best in respect to the peripheral mechanisms for pain, insofar as certain primary afferent fibers, namely the C and A-δ fibers and their free nerve ending receptors, respond maximally to noxious stimuli. Even these freely branching receptor endings and their pain fibers convey considerable non-noxious information; that is, their specificity as pain fibers is not absolute (Chap. 8). Nor has it been possible to ascribe a specific function to each of the many other types of receptors. Thus, Merkel discs and Meissner corpuscles within nerve plexuses around the hair follicles, and free nerve endings can all be activated by moving or stationary tactile stimuli. Conversely, a single type of receptor seems capable of generating more than one sensory modality. Lele and Weddell found that with appropriate stimulation of the cornea, each of the four primary modalities of somatic sensibility (touch, warmth, cold, pain) could be recognized, even though the cornea contains only fine, free nerve endings. In the outer ear, which is also sensitive to these four modalities, only two types of receptors—freely ending and perifollicular—are present. The lack of organized receptors—e.g., the end bulbs of Krause and Ruffini—in the cornea and ear makes it evident that these types of receptors are not essential for the recognition of cold and warmth as von Frey and other early anatomists had postulated.
Particularly instructive have been the observations of Kibler and Nathan, who studied the responses of warm and cold spots to different stimuli. (Warm and cold spots are those small areas of skin that respond most consistently to thermal stimuli with a sensation of warmth or cold.) They found that a cold stimulus applied to a warm spot gave rise to a sensation of cold and that a noxious stimulus applied to a warm or cold spot gave rise only to a painful sensation; they also noted that mechanical stimulation of these spots gave rise to a sensation of touch or pressure. These observations indicate that cutaneous receptors, some not easily distinguishable from one another on morphologic grounds, are probably endowed with only a relative degree of specificity, in the sense that each responds preferentially (i.e., has a lower threshold) to one particular form of stimulation. However, among the freely branching nociceptors there is some degree of specialization, as discussed in Chap. 8. Such end organs can then be classed as mechanoreceptors, thermoreceptors, or nociceptors, depending on their selective sensitivity to mechanical, thermal, or noxious stimuli respectively (Sinclair; Light and Perl).
Physiologic studies have shown that the quality of sensation depends on the type of nerve fiber that is activated. Different diseases therefore evoke a variety of sensory symptoms. Microstimulation of single sensory fibers in a peripheral nerve of an awake human subject arouses different sensations, depending on which fibers are stimulated and not on the frequency of stimulation. On the other hand, the frequency of stimulation governs the intensity of sensation, as does the number of sensory units that are stimulated. Stated somewhat differently, afferent impulse frequency (temporal summation) is encoded by the brain in terms of magnitude or intensity of sensation. In addition, as the intensity of stimulation increases, more sensory units are activated (spatial summation).
Localization of a stimulus was formerly thought to depend on the simultaneous activation of overlapping sensory units. Tower defined a peripheral sensory unit as a dorsal root ganglion cell, its central and distal processes, and all the sensory endings in the territory supplied by those distal processes (the receptive field of the sensory cell). In the very sensitive pulp of the finger, where the error of localization is less than 1 mm, there are 240 overlapping, low-threshold mechanoreceptors per square centimeter. Highly refined physiologic techniques have demonstrated that activation of even a single sensory unit is sufficient to localize the point stimulated and that the body map in the parietal lobe is capable, by its modular columnar organization, of encoding such refined topographic information. Also, each point in the skin that is stimulated may involve more than one type of receptor. To gain access to its receptor, a stimulus must pass through the skin and possess sufficient energy to transduce, i.e., depolarize, the nerve ending.
Not only does the threshold of stimulation vary but the nerve impulse that is generated is a graded one, not an all-or-none phenomenon like an action potential in nerve. This poorly understood peripheral generator potential determines the freqsuency of impulses in the nerve and to what degree it is sustained or fades out (i.e., adapts to the stimulus or fatigues). However, the mechanism by which a stimulus is translated into a sensory experience, i.e., is encoded, is now partially understood. Special molecules transduce physical alterations by opening cationic channels. As mentioned in Chap. 8, these changes lead to the opening of voltage-gated sodium channels, which generates an action potential. It is probably fair to say that each type of specialized ending has a membrane structure that facilitates the transduction process for a particular type of stimulus. In general, the encapsulated endings, which are more highly myelinated, are of low-threshold type, variably adaptable to continued stimulation (Meissner and pacinian corpuscles are rapidly adapting; Merkel discs and Ruffini endings are slowly adapting) and are connected to large sensory fibers (see Lindblom and Ochoa). The pacinian receptors are the most deeply situated (Fig. 9-1).
Fibers that mediate superficial sensation are located in cutaneous sensory or mixed sensorimotor nerves. In cutaneous nerves, unmyelinated pain and autonomic fibers exceed myelinated fibers by a ratio of 3 or 4:1. The myelinated fibers are of two types: small, lightly myelinated, A-δ fibers for pain and cold, as discussed in Chap. 8, and larger, faster-conducting A-α fibers for touch and pressure. Nonmyelinated autonomic fibers are efferent (postganglionic) and innervate piloerector muscles, sweat glands, and blood vessels. The differing conduction velocities of the fibers of these are discussed in Chap. 45.
A single cutaneous (touch, pain, and temperature) type of afferent fiber connects to several receptors, all of one type, which are irregularly distributed in the skin and account for sensory spots. Stimuli from a given spot are therefore conveyed by two or more fibers. The proprioceptive fibers subserve pressure sense and, with endings in articular structures, the sense of position and movement; they enable one to discriminate the form, size, texture, and weight of objects. Sensations of tickle, itch, and wetness are believed to arise from combinations of several types of receptors.
Itch is a distinctive sensation that can be separated on clinical and neurophysiologic grounds from touch and from pain. Two aspects are recognized: a brief primary localized itch at the site of stimulus or injury, and a subsequent more diffuse sensation that is greatly intensified by gentle touch. Itch sensation is transmitted by specific C fibers, not by touch mechanisms, for regions of analgesia no longer can be stimulated to itch but areas anesthesia retain this sensation. There is, however, no specialized peripheral itch receptor, the sensation depending instead on the spinal connections to itch pathways. Several forebrain regions are activated by itch, i.e., there is no central “itch center.” From the perspective of neurologic diseases, the main cause of itch is postherpetic neuralgia, but cases of paroxysmal itch of central origin are known to be caused by multiple sclerosis. The pathophysiology of itching has been discussed by Greaves and Wall and is reviewed further by Yosipovitch and colleagues.
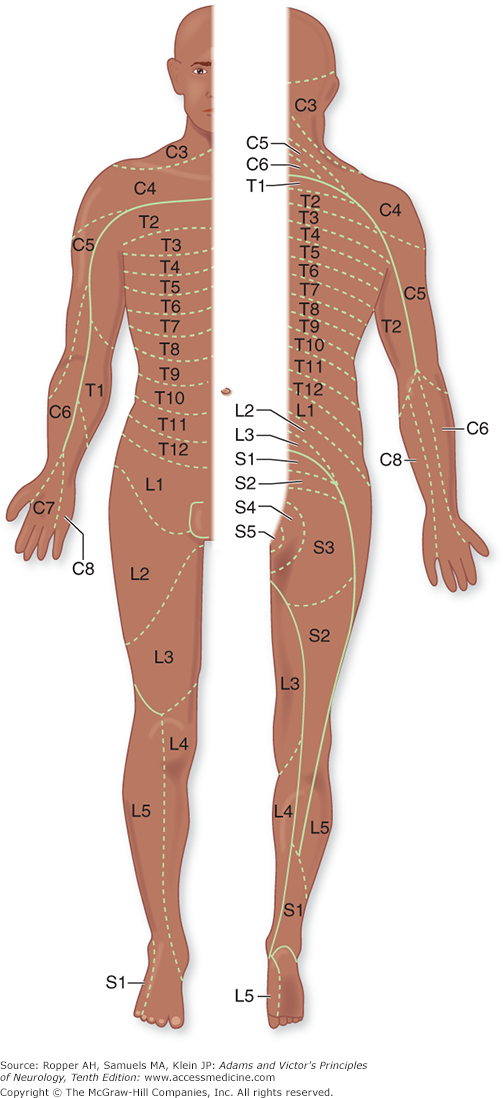
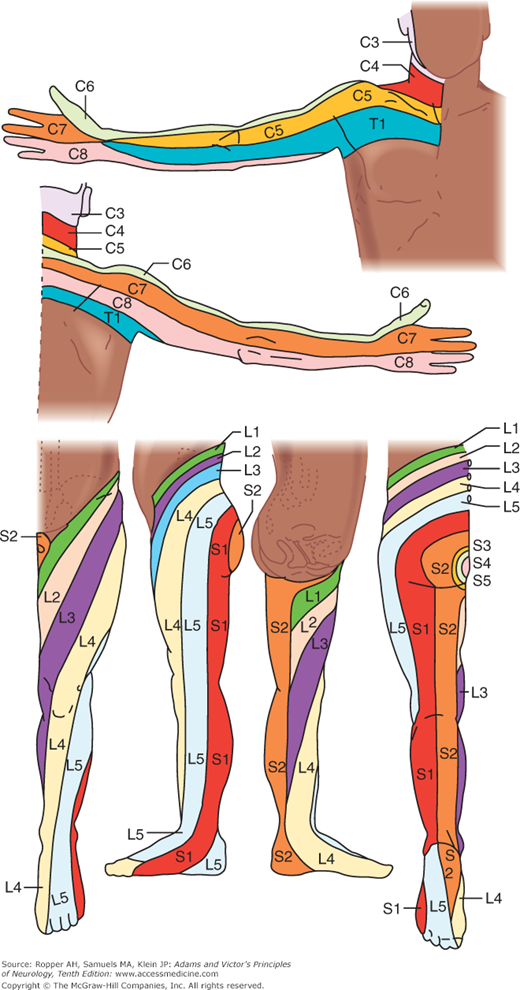
All the sensory neurons have their cell bodies in the dorsal root ganglia. The peripheral extensions of these cells constitute the sensory nerves; the central projections of these same cells form the posterior (dorsal) roots and enter the spinal cord. Each dorsal root contains all the fibers from skin, muscles, connective tissue, ligaments, tendons, joints, bones, and viscera that lie within the distribution of a single body segment (somite). This segmental innervation has been amply demonstrated in humans and animals by observing the effects of lesions that involve one or two spinal nerves, such as (1) herpes zoster, which also causes visible vesicles in the corresponding area of skin; (2) the effects of a prolapsed intervertebral disc, which causes hypalgesia in a single root zone; and (3) surgical section of several roots on each side of an intact root (method of remaining sensibility). Maps of the dermatomes derived from these several types of data are shown in Figs. 9-3 and 9-4. It should be noted that there is considerable overlap from one dermatomal segment to the other, more so for touch than for pain. By contrast, there is less overlap between adjacent peripheral nerves and almost none between the divisions of the trigeminal nerve. Also, the maps differ somewhat according to the methods used in constructing them. In contrast to most dermatomal charts, those of Keegan and Garrett (based on the injection of local anesthetic into single dorsal root ganglia) show bands of hypalgesia to be continuous longitudinally from the periphery to the spine (Fig. 9-4). The distribution of pain fibers from deep structures, although not exactly corresponding to that of pain fibers from the skin, also follows a segmental pattern. In Chap. 8 it was commented that the areas of projection of referred pain from the visceral organs and musculoskeletal structures roughly correspond to the overlying dermatomes but they have distinctive patterns, termed sclerotomes (Fig. 8-5).
Figure 9-3.
Distribution of the sensory spinal roots on the surface of the body (dermatomes). (Reproduced by permission from Sinclair.)
Figure 9-4.
Dermatomes of the upper and lower extremities, outlined by the pattern of sensory loss following lesions of single nerve roots. (Reproduced by permission from Keegan and Garrett.)
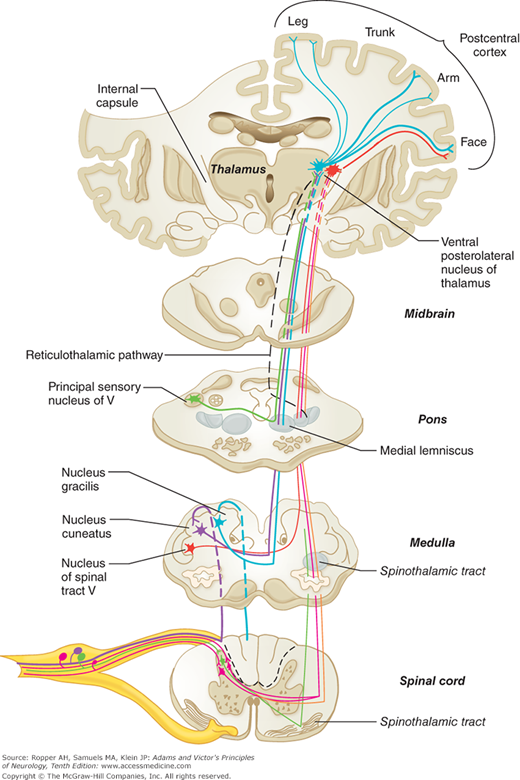
In the dorsal roots, the sensory fibers are first rearranged according to function. Large and heavily myelinated fibers enter the cord just medial to the dorsal horn and divide into ascending and descending branches. The descending fibers and some of the ascending ones enter the gray matter of the dorsal horn within a few segments of their entrance and synapse with nerve cells in the posterior horns as well as with large ventral horn cells that subserve segmental reflexes. Some of the ascending fibers run uninterruptedly in the dorsal columns of the same side of the spinal cord, terminating in the gracile and cuneate nuclei in the upper cervical spinal cord and medulla (Fig. 9-5). The central axons of the primary sensory neurons are joined in the posterior columns by other secondary neurons whose cell bodies lie in the posterior horns of the spinal cord (see below). The fibers in the posterior columns assume a medial position as new fibers from each successively higher root are added laterally, thereby creating somatotopic laminations (see Fig. 8-3).
Figure 9-5.
The main somatosensory pathways emphasizing the posterior column–lemniscal system (thicker tract lines). See Fig. 8-2 for comparison with the spinothalamic system.
Of the long ascending posterior column fibers, which are activated by mechanical stimuli of skin and subcutaneous tissues and by movement of joints, only about 25 percent (from the lumbar region) reach the gracile nuclei at the upper cervical cord. The remaining fibers send collaterals to, or terminate in, the dorsal horns of the spinal cord, at least in the cat (Davidoff). An estimated 20 percent of ascending fibers originate from cells in Rexed layers IV and V of the posterior horns (see Fig. 8-1) and convey impulses from low-threshold mechanoreceptors that are sensitive to hair movement, skin pressure, or noxious stimuli. There are also descending fibers in the posterior columns, including fibers from cells in the dorsal column nuclei.
The posterior columns contain a portion of the fibers for the sense of touch as well as the fibers mediating the senses of touch-pressure, vibration, direction of movement and position of joints, and stereoesthesia—recognition of surface texture, shape, numbers, and figures written on the skin and two-point discrimination—all of which depend on patterns of touch-pressure (see Fig. 8-2). The nerve cells of the nuclei gracilis and cuneatus and accessory cuneate nuclei give rise to a secondary afferent path, which crosses the midline in the medulla and ascends as the medial lemniscus to the posterior thalamus. However, the fiber pathways in the posterior columns are not the sole mediators of proprioception in the spinal cord (see “Posterior [Dorsal] Column Syndrome” further on).
In addition to the well-defined posterior column pathways, there are cells in the more loosely structured “reticular” part of the dorsal column that receive secondary ascending fibers from the dorsal horns of the spinal cord and from ascending fibers in the posterolateral columns. These dorsal column fibers project to brainstem nuclei, cerebellum, and thalamic nuclei. Many other cells of the dorsal horn nuclei are interneurons, with both excitatory and inhibitory effects on local reflexes or on the primary ascending neurons. They are also under the influence of the sensorimotor cortex. Some act on descending corticospinal motor neurons. The functions of many of the extrathalamic projections of dorsal column cells are unknown (Davidoff).
Thinly myelinated or unmyelinated fibers, subserving mainly pain sensibility, but some sensitive to touch and pressure, enter the cord on the lateral aspect of the dorsal horn and synapse with dorsal horn cells, mainly within a segment or two of their point of entry into the cord. The dorsal horn cells, in turn, give rise to secondary sensory fibers, some of which may ascend ipsilaterally but most of which decussate and ascend in the spinothalamic tracts, as described in Chap. 8 (see Figs. 8-1 and 8-2). Observations based on surgical interruption of the anterolateral funiculus indicate that fibers mediating touch and deep pressure occupy the ventromedial part (anterior spinothalamic tract). Also as remarked in Chap. 8, an ascending tract of secondary sensory axons lies in or medial to the descending corticospinal system.
After the posterior columns terminate in the gracile and cuneate nuclei of the rostral cervical cord and medulla, synapses are made with fibers that cross the midline and ascend to form the medial lemniscal tracts in the brainstem. The lemniscal system is situated in a paramedian position, changing orientation slightly at different levels of the brainstem, and joining the spinothalamic system in the rostral midbrain to terminate in the posterior thalamic nuclei (see below and also Fig. 8-2).
The pathways mediating cutaneous sensation from the face and head—especially touch, pain, and temperature—are conveyed to the brainstem by the trigeminal nerve. After entering the pons, the pain and temperature fibers turn caudally and run through the ipsilateral medulla as the descending spinal trigeminal tract, terminating in the long, vertically oriented nucleus caudalis, or spinal trigeminal nucleus, that lies beside it and extends to the second or third cervical segment of the cord, where it becomes continuous with the posterior horn of the spinal gray matter. Axons from the neurons of this nucleus cross the midline and ascend as the trigeminal quintothalamic tract (also termed, somewhat imprecisely, trigeminal lemniscus) along the medial side of the spinothalamic tract (see Fig. 8-2), of which it is the equivalent.
The ventral posterior thalamic nucleus receives fibers from the medial lemniscal, spinothalamic, and trigeminal (fibers from the principal sensory and spinal trigeminal nuclei) tracts, and projects mainly to two somatosensory cortical areas. The first area (S1) corresponds to the post-central cortex or Brodmann areas 3, 1, and 2. S1 afferents are derived primarily from the ventral posterolateral nucleus (VPL, the terminus of medial lemniscal and spinothalamic fibers) and the ventral posteromedial nucleus (VPM, the terminus of trigeminal fibers) and are distributed somatotopically, with the leg represented uppermost and the face lowermost (face and hand are juxtaposed). Electrical stimulation of this area yields sensations of tingling, numbness, and warmth in specific regions on the opposite side of the body. The information transmitted to S1 is tactile and proprioceptive, derived mainly from the dorsal column–medial lemniscus system and concerned mainly with sensory discrimination. The second somatosensory area (S2) lies on the upper bank of the sylvian fissure, adjacent to the insula. Localization of function is less discrete in S2 than in S1, but S2 is also organized somatotopically, with the face rostrally and the leg caudally. The sensations evoked by electrical stimulation of S2 are much the same as those of S1 but, in distinction to the latter, may be felt bilaterally. Of note is the failure of cortical stimulation to evoke pain sensation.
Undoubtedly, the perception of sensory stimuli involves more of the cerebral cortex than the two discrete areas described above. Some sensory fibers probably project to the precentral gyrus and others to the superior parietal lobule. Moreover, S1 and S2 are not purely sensory in function; motor effects can be obtained by stimulating them electrically. It has been shown that sensory neurons in VPL, cuneate and gracile nuclei, and sensory neurons in the dorsal horns of the spinal cord all receive descending as well as ascending cortical projections. This reciprocal arrangement probably influences movement and the transmission and interpretation of pain, as discussed in Chap. 8.
Provided that the subcortical structures, especially the thalamus, are intact, certain sensations such as pain, touch, pressure, and extremes of temperature can reach consciousness. Their accurate localization, however, as well as the patient’s ability to make other fine sensory discriminations, depends to a large extent on the integrity of the sensory cortex. This clinical distinction is elaborated in the discussion of the sensory syndromes further on.
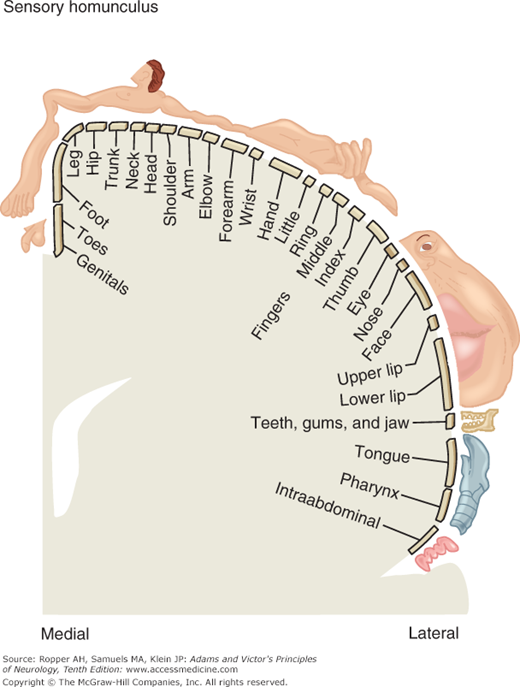
Figure 9-6 (“sensory homunculus”) shows the cortical representation of sensory information in the postcentral gyrus. As in the case of the motor representation (see Fig. 3-4), a disproportionate area is devoted to localization in the fingers, lips, and face.
Figure 9-6.
The “sensory homunculus,” or cortical representation of sensation in the postcentral gyrus; compare this to the distribution of body areas in the motor cortex as shown in Fig. 3-4. (Reproduced with permission from Kandel ER, Schwartz JH, Jessel TM: Principle of Neural Science, 4th ed. New York, McGraw-Hill, 2000.)
From this brief account of the various channels of sensory information, one must conclude that at every level there is the possibility of feedback control from higher levels. Most external and some internal stimuli are highly complex and induce activity in more than one sensory system. In every system there is sufficient redundancy to allow lesser-used systems to compensate partially for the deficits incurred by disease.
Examination of Sensation
Most neurologists would agree that sensory testing is the most difficult part of the neurologic examination. For one thing, test procedures are relatively crude and are unlike those natural modes of stimulation with which the patient is familiar. It is also fair to say that few diagnoses are made solely on the basis of the sensory examination; more often the exercise serves to complement the motor examination. Quite often, no objective sensory loss can be demonstrated despite symptoms that suggest the presence of such an abnormality. Only rarely does the opposite pertain, i.e., one discovers a sensory deficit when there has been no complaint of sensory symptoms. Sensory symptoms in the nature of paresthesia or dysesthesia may be generated along axons of nerves not sufficiently diseased to impair or reduce sensory function; in the latter instance, loss of function may have been so mild and gradual as to pass unnoticed. Always there is some difficulty in evaluating the response to sensory stimuli, as it depends on the patient’s interpretation of sensory experiences. This, in turn, will depend on the patient’s general awareness and responsiveness and ability to cooperate, as well as degree of suggestibility. Children, by virtue of their simple and direct responses, are often better witnesses than more sophisticated individuals who are likely to analyze their feelings minutely and report small and insignificant differences in stimulus intensity.
Before proceeding to sensory testing, the physician should question patients about their symptoms; this, too, poses particular problems. Patients may be confronted with derangements of sensation that are unlike anything they have previously experienced, and they have limited terms to describe what they feel. They may say that a limb feels “numb” and “dead” when in fact they mean that it is weak. Occasionally a loss of sensation is discovered almost accidentally, e.g., by a lack of pain on touching an object hot enough to blister the skin or unawareness of articles of clothing and other objects in contact with the skin. More often, disease induces new and unnatural sensory experiences such as a band of tightness, a feeling of the feet being encased in cement, lancinating pains, an unnatural feeling when stroking the skin, a sensation as if walking on pebbles, and so on.
If nerves, sensory roots, or spinal tracts are damaged or partially interrupted, the patient may complain of tingling or prickling feelings (“like Novocain” or like the feelings in a limb that has “fallen asleep,” the common colloquialism for nerve compression), cramp-like sensations, or burning or cutting pain occurring either spontaneously or in response to stimulation.
Experimental data support the view that partially damaged touch, pressure, thermal, and pain fibers become hyperexcitable and generate ectopic impulses along their course, either spontaneously or in response to a natural volley of stimulus-evoked impulses (Ochoa and Torebjork). These abnormal sensations are experienced as paresthesias, or dysesthesias if they are severe and distressing, as noted in Table 8-2. Another positive sensory symptom is allodynia, referring to a phenomenon in which one type of stimulus evokes another type of sensation—e.g., touch may induce pain.
The clinical description of a sensation may divulge the particular sensory fibers involved (Table 9-1). It is known that stimulation of touch fibers gives rise to a sensation of tingling and buzzing; of muscle proprioceptors, to pseudocramp (the sensation of cramping without actual muscle contraction); of thermal fibers, to heat (including burning) and coldness; and of A-δ fibers, to prickling and pain. Paresthesia arising from ectopic discharges in large sensory fibers can be induced by nerve compression, hypocalcemia, hypomagnesemia, certain medications (niacin foremost among them), and diverse diseases of nerves. Band-like sensations on a limb or the trunk are the result of dysfunction in large sensory fibers, either in the periphery or their continuation in the posterior columns. Certain sensory symptoms suggest an anatomic location of nerve disease; for example, lancinating pains that radiate to the back or neck implicate root or, less often, sensory ganglion disease.
SYMPTOMS | STRUCTURES AFFECTED |
|---|---|
Paresthesia, tingling, buzzing | Large fibers (in nerve or posterior columns) |
Burning, heat, cold | Small fibers |
Prickling pain | Combined small- and large-fiber |
Pseudocramp | A type of paresthesia, probably related to large-fiber dysfunction |
Band tightness | Lemniscal system of cord |
Lancinating pain | Small-fiber neuropathy and radiculopathy |
Hyperalgesia |