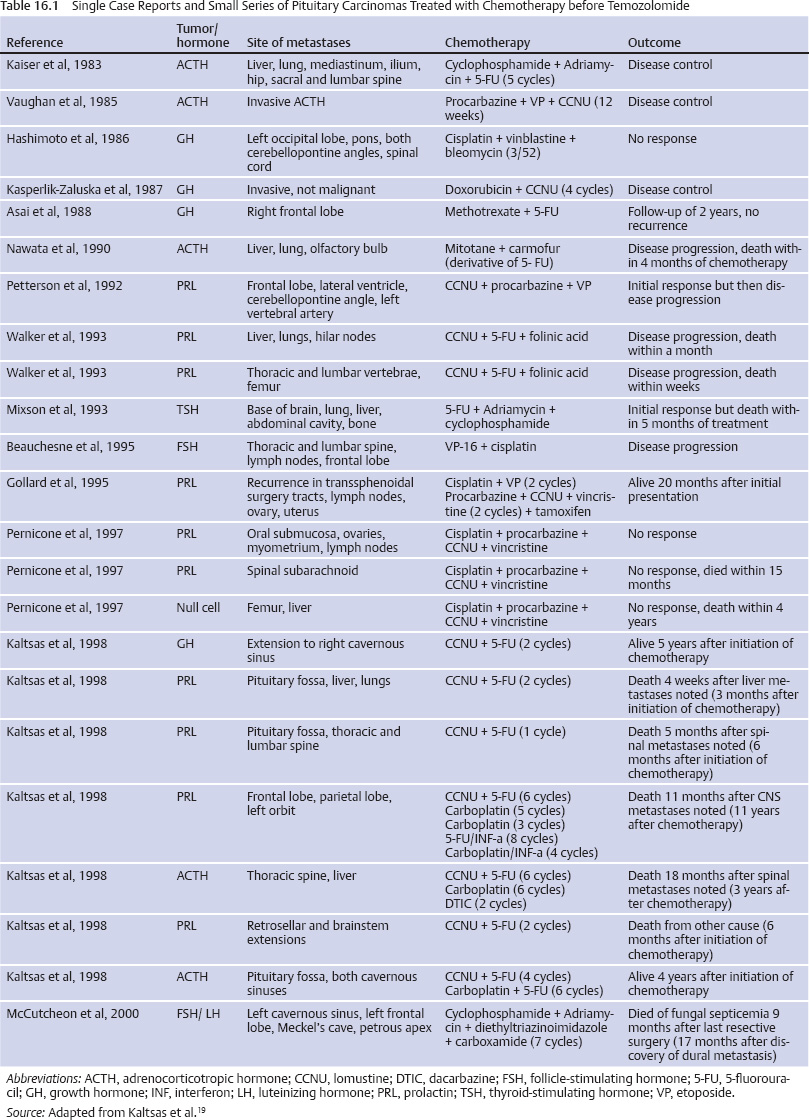
16 Chemotherapy Options for Sellar and Parasellar Tumors Among the sellar and parasellar tumors that may be amenable to treatment with chemotherapy are pituitary carcinomas, craniopharyngiomas, germ cell tumors, chordomas, plasmacytomas, meningiomas, and primary central nervous system (CNS) lymphomas of the pituitary. Even though these tumors all together account for a very small fraction of intracranial neoplasms, they deserve special attention because of their location, which may result in endocrine and visual disturbances. Pituitary carcinomas are extremely rare and aggressive tumors, accounting for only 0.1 to 0.2% of all pituitary tumors.1–3 The hallmark of these tumors is concomitant metastasis, either outside the CNS or in noncontiguous foci within the CNS. The mainstay of the treatment of pituitary carcinoma remains surgery, with either conventional or focused adjuvant radiation therapy modalities that have been well discussed in other chapters of this book. However, when the tumor is a carcinoma, disease generally progresses despite these interventions. Cytotoxic chemotherapy is usually employed as a last resort, and several single case reports and small series of pituitary carcinomas treated with chemotherapy have been published2,4–18 (Table 16.1). Temozolomide (TMZ) is an imidazotetrazine derivative that acts as a DNA-methylating agent. It is absorbed rapidly after oral administration and crosses the blood-brain barrier.20 TMZ in addition to radiation therapy is the standard treatment for glioblastoma (GBM).21,22 The effect of TMZ depends on methylation of a specific guanine in DNA. MGMT (O-6-methylguanine-DNA methyltransferase) is a DNA repair enzyme removing the alkyl group from the guanine.23,24 A high level of expression of MGMT in gliomas implicates a poor response to TMZ.23 McCormack et al, analyzing MGMT expression in 88 pituitary tumor samples, found low MGMT expression in only 13% of the cases and did not find a significant difference between MGMT expression in invasive and noninvasive tumors, or in recurrent and nonrecurrent tumors.25 TMZ is usually administered at a dose of 200 mg/m2 per day for 5 days in cycles of 28 days, as for high-grade gliomas. Recently, a multicenter Phase II study by the Spanish Neuro-Oncology Group using extended-schedule dose-dense TMZ in refractory gliomas (85 mg/m2 per day for 21 consecutive days in 28-day cycles until disease progression or unacceptable toxicity) showed activity deserving further evaluation in larger cohorts.26 The rationale for the protracted schedule is the continuous depletion of MGMT, enhancing the cytotoxicity of TMZ.27,28 Fadul et al in 2004 reported in abstract form two cases of pituitary carcinoma that responded partially to TMZ. The first patient, who had a prolactin-secreting tumor, received 10 cycles of TMZ with reduction of prolactin levels and control of pain caused by metastasis to the cervical and thoracic spine; treatment was stopped secondary to persistent fatigue. The second patient, who had a nonsecreting pituitary carcinoma and local invasion plus multiple drop metastases to the cervical and thoracic spinal cord, received 12 cycles of TMZ with improvement of visual fields and magnetic resonance imaging (MRI) showing reduction of the metastasis volume until treatment was discontinued secondary to lymphopenia.29 Also in 2004, Zhu et al reported the successful use of TMZ in a patient with refractory malignant prolactinoma.30 Another reported case summarized the course of a 72-year-old man who had a prolactin-secreting pituitary carcinoma treated with TMZ during 24 months, with stabilization of his prolactin levels, marked reduction of the size of the cerebellopontine angle and cervical metastatic deposits, and a remarkable functional recovery.31 Kovacs et al reported a case of a prolactin-secreting pituitary carcinoma treated with TMZ with decrease of serum prolactin levels and clinical improvement.32 Kovacs et al also demonstrated hemorrhage, necrosis, fibrosis, and shrinkage in the tumor after TMZ treatment. Neuronal transformation was also seen, a process described in various tumor types including pituitary adenomas, mainly growth hormone (GH)–producing tumors.33,34 The cause of the neuronal transformation is unknown, and the authors believe that TMZ may act similarly to nerve growth factor, causing neuronal metaplasia, which is the transformation of pituitary tumor cells to nerve cells. Hagen et al reported two patients with invasive pituitary macroadenoma and one patient with pituitary carcinoma treated with TMZ who experienced a significant decrease in tumor volume, hormone hypersecretion, and symptoms. All three patients were tested for MGMT expression; this was negative in two patients, and only a few nuclei stained positive in the third patient, potentially explaining the favorable treatment response.24 Neffet al reported a patient who had an invasive prolactinoma treated with TMZ, with marked decrease and stabilization of the prolactin levels and continuous tumor bulk reduction on follow-up imaging over 26 cycles of TMZ.35 Moyes et al reported a case of a patient with Nelson syndrome, an aggressive ACTH-secreting macroadenoma, in addition to high levels of ACTH and skin hyperpigmentation, treated successfully with TMZ. The ACTH levels fell from 2472 to 389 pmol/L, and follow-up imaging confirmed marked shrinkage of the tumor after 4 cycles of TMZ.36 Immunostaining was negative for MGMT, again perhaps explaining the favorable tumor response. Mohammed et al published three cases of aggressive pituitary macroadenomas treated with TMZ. The first two patients had ACTH-secreting macroadenomas of the Crooke’s cell variant, and the third patient harbored GBM with an incidental pituitary tumor. The first two patients had radiologic evidence of tumor shrinkage and clinical improvement during treatment. One of these patients had a diagnosis of Nelson syndrome. The third patient underwent radiation therapy and TMZ for GBM, and pituitary tumor reduction was also noticed on follow-up scans, with collapse of the suprasellar component into the sella despite the sellar area not having been included in the radiation treatment field.37 Recently, Thearle et al reported a patient with an invasive ACTH-secreting macroadenoma who failed conventional treatment and developed rapid tumor growth leading to the compromise of multiple cranial nerves. This patient was treated with a combination of TMZ and capecitabine (Xeloda), with a marked tumor burden reduction and a decrease in ACTH levels of more than 90%. The tumor recurred after 5 months with a hypermetabolic positron emission tomography (PET) scan, suggesting transformation into a more aggressive histology.38 Capecitabine is an oral chemotherapeutic agent that is converted to 5-fluorouracil (5-FU) in vivo by the enzyme thymidine phosphorylase.39 5-FU is metabolized to 5-dUMP, which produces a deficiency of thymidylate by inhibiting thymidylate synthase (TS). This leads to a decrease in DNA synthesis and inhibition of cell division by reducing the conversion of UMP to dTMP by TS, leading to thymidylate deficiency. TMZ causes DNA damage at any point in the cell cycle through base pair mismatch of O-6-methylguanine with thymidine in the sister chromatid instead of cytosine, which arises because the mismatch repair enzymes misread the methylated guanine as an adenosine and place thymidine in the sister chromatid. The basis of the use of capecitabine for 10 days before the addition of TMZ is that decreased thymidine levels intracellularly accentuate the mismatch repair process leading to a break in DNA, which is a potent stimulus for apoptosis.38,39 Bush et al reported a retrospective series of seven patients with aggressive pituitary tumors treated with TMZ.40 MGMT promoter methylation and MGMT expression in 14 surgical specimens from these seven patients were compared and correlated with the clinical response to TMZ. Clinically significant tumor reduction was observed in two patients, a 20% tumor reduction in one, stable disease in three, and progressive metastatic disease in one patient. The DNA promoter site for MGMT was unmethylated, and variable amounts of MGMT expression were seen in all 14 specimens. The authors could not find any correlation between MGMT expression and clinical outcomes but concluded that TMZ is a valuable alternative in the management of aggressive pituitary tumors. Laws et al in 2003 published the results of a Phase I study of transsphenoidal post-resection implantation of Gliadel wafers in nine patients with aggressive pituitary adenomas.41 Gliadel is a polymer wafer impregnated with BCNU (bis-chloroethyl-nitrosurea), an alkylating agent, designed to capitalize on the concept of delivering local treatment to brain tumors, avoiding the toxic effects of systemic treatment and bypassing the blood-brain barrier.42,43 Three patients were disease-free after a mean follow-up of 19 months, two had stable residual disease, one had disease progression, two patients died secondary to disease progression, and one died because of a stroke. The study concluded that Gliadel implantation into the sella turcica for aggressive pituitary tumors is safe.41 No further studies of this agent in pituitary adenomas have been reported. With respect to ongoing studies, presently there is one Phase II study evaluating the safety and efficacy of TMZ in the treatment of invasive pituitary tumors (NCT00601289). In addition, there is one study investigating the use of lapatinib in slowing the growth rate of pituitary tumors. Lapatinib is an FDA-approved drug used to treat breast cancer and has been shown to inhibit both epidermal growth factor receptor (EGFR) and erbB2 tyrosine kinases, which are overexpressed in pituitary adenomas. Craniopharyngiomas are benign tumors of the sellar and parasellar region that may be solid, cystic, or a combination of the two. Their treatment poses a challenge because the tumors are difficult to completely resect, and recurrence, even in instances of presumed gross total resection, is common. Craniopharyngiomas are the most common suprasellar tumor in the pediatric age group and the most common nonglial primary intracranial tumor in children.44,45 The treatment of craniopharyngiomas is multidisciplinary, involving surgical resection by craniotomy or the transsphenoidal route, fractionated radiation therapy, and radiosurgery for residual tumor.44 In patients with primarily cystic tumors, recurrent tumors, and/or tumors with a difficult surgical approach, and in young patients, for whom postponing more aggressive treatment such as radiation therapy or surgery is desirable, the use of intracavitary bleomycin has a role.46 Bleomycin, an antibiotic produced from the fungus Streptomyces verticillus, inducessingle-strand and double-strand DNA breaks.46–48 The double-strand breaks and resulting loss of chromosome fragments probably account for bleomycin cytotoxicity.46,49 Takahashi et al in 1985 reported the first seven cases of craniopharyngiomas treated with intracavitary bleomycin administered through an Ommaya reservoir inserted into the cyst.50 Among these patients, those with cystic tumors had a better outcome than the ones with solid or combination lesions.50 This initial publication was supported by several others.51–60 Hargrave in 2006, analyzing the data of those publications, found that stable disease (stable cyst volume reduction) was achieved in 57% of the patients, with an obviously better response seen in cystic craniopharyngiomas. The median dose of bleomycin was 5 mg (2–15 mg). Seventyone percent of the patients received 3 doses per week (1–7 doses); the median number of doses was 8 (1–30). The median cumulative dose was 53 mg (5–150 mg).61 The major side effects directly attributed to bleomycin were headaches (42%), fever (36%), nausea and vomiting (21%), endocrine manifestations (16%), somnolence (15%), infarct/hemorrhage (10%), personality changes, vision changes (7%), and hearing problems, seizures, and death related to bleomycin therapy (1%).61 Hukin et al recently reported the Canadian experience with intracystic bleomycin.62 Seventeen patients were reported, of whom 12 were treated at the time of diagnosis and five at recurrence. Five patients achieved a complete response and six a partial response, and five had a minor response to intracystic bleomycin. The treatment protocol varied between centers, with most following a thriceweekly regimen. The course of treatment averaged 4 weeks (range, 2–15 weeks), and the median dose per course was 36 mg (range, 15–75 mg). The median dose per instillation was 3 mg (range, 2–5 mg), and the median dose per week was 9 mg (range, 4–20 mg). In a single injection, the median drug concentration within the cyst was 0.09 mg/ mL per dose (range, 0.01–2 mg/mL per dose). The median follow-up was 4 years (range, 0.5–10.2 years), and the median progression-free survival (PFS) was 1.8 years (range, 0.3–6.1 years). Observed complications included transient symptomatic peritumoral edema in two patients, precocious puberty in one patient, and panhypopituitarism in two patients. Bleomycin is a neurotoxic drug; if leakage occurs, severe complications may develop, such as hypothalamic toxicity causing hypersomnia, personality changes, memory impairment, and thermal dysfunction,60 and even death.57 Lactate dehydrogenase (LDH) activity in craniopharyngioma fluid is elevated typically to ~3000 units, and the LDH isoenzyme pattern shows elevation of L4 and L5 fractions. Repeated injection of bleomycin causes a gradual clearing of the motor oil–colored cystic fluid, with a decrease in LDH activity (<1000 units), and the L4 and L5 fractions show a flat pattern.50,63 Laws et al treated one patient who had craniopharyngioma with Gliadel placement after transsphenoidal tumor resection. This patient had previously undergone three transsphenoidal resections, one craniotomy, and one gamma knife radiosurgery; at recurrence, the patient was treated with a new transsphenoidal tumor resection and placement of three Gliadel wafers. After 6 months of follow-up, no tumor recurrence was seen.41 Jakacki et al reported a Phase II evaluation of systemic interferon alfa-2a (IFN-α-2a) in patients with progressive and/or recurrent craniopharyngiomas.64 The rationale for using IFN-α-2a is that the drug shows activity against squamous cell skin carcinoma, which is believed to have the same embryologic origin as craniopharyngioma.65,66 IFNs exert antitumor activity through direct antiproliferative, cytotoxic, and maturational effects, and they also modulate the immune response.64,67,68 IFN-α-2a was administered in a dose of 8 million units (MU) per square meter daily for 16 weeks (induction phase), followed by the same dose three times per week for 32 weeks (maintenance phase). Fifteen patients received the drug, but only 12 could be followed with imaging. Radiologic response was seen in three patients with predominantly cystic tumors. Intratumoral IFN-α was used in nine patients, with complete disappearance of the tumor in seven and partial reduction in two. Mean follow-up was 1 year and 8 months (range, 1–3.5 years), and the doses ranged from 18 to 36 MU. The most frequent side effects of IFN-α are fatigue, fever, weight loss, loss of appetite, and behavioral changes.59 IFN-α also seems to reduce the size of cystic craniopharyngiomas by activating the Fas apoptotic pathway. Twenty-one patients with cystic craniopharyngiomas received intracystic IFN-α; the tumor size of all patients and the apoptotic factor soluble FasL (sFasL) concentration in eight patients were monitored. A complete response (tumor reduction) was observed in 11 patients (52.4%), a partial response in seven (33.3%), and a minor response in three (14.3%). The concentration of sFasL was increased in all eight patients, and it was examined concomitantly with tumor reduction. The mean follow-up was 27 months (range, 6 months to 4 years). Two patients underwent surgical resection, and tumor removal was easier than in the ones who had received chemotherapy preoperatively69 Although particularly rare, primary central nervous system lymphomas (PCNSLs) arising from the sellar and parasellar region are one of the causes of hypothalamic-pituitary dysfunction.70 In a series of 1120 patients with pituitary tumors resected via the transsphenoidal route, only one case (0.1%) was diagnosed as a pituitary lymphoma.71 In another series of 353 patients with pituitary masses, pituitary lymphoma was found in one case (0.3%).72 The majority of PCNSLs are B-cell lymphomas, and besides acquired immunodeficiency syndrome (AIDS) and other immunodeficiency conditions, lymphocytic hypophysitis and pituitary adenomas have been implicated as risk factors for pituitary lymphomas.73 The treatment of PCNSL traditionally combines whole-brain radiation therapy (WBRT) and chemotherapy with methotrexate (MTX) alone or in combination with other agents. MTX is a folate antagonist and has limited penetration in the CNS because of its high degree of ionization at a physiologic pH; high doses (≥3.5 g/m2) are used to achieve cytotoxic intratumoral concentrations.74 We extrapolate the treatment of PCNSL anywhere in the CNS to lesions arising in the sellar and parasellar areas. Several combination regimens of WBRT and MTX are associated with reasonable response rates.75–80 The combination of MTX, procarbazine, and vincristine (MPV) followed by WBRT and cytarabine after radiation is associated with an overall response rate of 91%, a PFS of 24 months, and an overall survival of 36.9 months.81 In another important study, rituximab, a chimeric monoclonal antibody against the protein CD20, which is primarily found on the surface of B cells, was tested with MPV followed by a lower dose of WBRT (23.4 Gy for patients who had complete response to chemotherapy or 45 Gy for patients who did not have a complete response).77 The overall response rate was 93%, and the 2-year median PFS rate was 57%. No treatmentrelated neurotoxicity was observed. The authors concluded that the addition of rituximab to MPV (R-MPV) increased the risk for significant neutropenia requiring routine growth factor support, that additional cycles of R-MPV nearly doubled the complete response rate, and that reduced-dose WBRT was not associated with neurocognitive decline. Chemotherapy protocols without WBRT were designed to avoid the delayed cognitive effects associated with radiation therapy, particularly in patients older than 60 years of age and in those with underlying vascular risk factors, with radiation therapy reserved for an eventual relapse.74 Intravenous MTX alone, 8 g/m2, was used in 25 patients, with a complete response achieved in 52% of the patients, a median PFS of 12.8 months, and a median overall survival of 55.4 months.82,83 Interestingly, 5 of 25 patients in this trial treated with MTX alone achieved a complete response and, after a median follow-up of 6.8 years, had not had a relapse. Several other drugs, including rituximab, have been added to MTX therapy in regimens that do not include WBRT,84,85 with the intent to achieve a more durable response. The blood-brain barrier can limit the diffusion of MTX into brain and tumor. The blood-brain barrier can be disrupted with the use of osmotic agents (ie, mannitol), enhancing drug delivery to the tumor. A multicenter analysis of 149 patients treated with blood-brain barrier disruption and intra-arterial MTX over 23 years showed an overall response rate of 81.9% (57.8% complete response), a median overall survival of 3.1 years (25% estimated survival at 8.5 years), a median PFS of 1.8 years, a 5-year PFS rate of 31%, and a 7-year PFS rate of 25%.86 Focal seizures (9.2%) were the most frequent side effect, and no long-term sequelae were reported. The study concluded that tumor control and outcomes were comparable or superior to those achieved with other PCNSL treatment regimens. Intrathecal chemotherapy is reserved for patients with concomitant leptomeningeal lymphoma, although prospective87–89 and retrospective90,91 studies indicate that intrathecal chemotherapy does not improve outcomes in patients who received MTX-based therapy.75 Additionally, some studies indicate that systemic MTX eradicates neoplastic cells from the cerebrospinal fluid (CSF).92,93 Other aspects should be taken into consideration before intrathecal chemotherapy is started, including the risks for complications of Ommaya reservoir placement, chemical meningitis, drug leakage outside the CNS, and infection. Several clinical trials have included intrathecal chemotherapy.78,79,84,85 Patients with relapse of PCNSL after an initial response to therapy have a median survival of ~4.5 months, posing a challenge to the clinician. WBRT alone is associated with a radiographic response in 74 to 79% of patients, with a median survival after radiation of 10.9 to 16 months; patients younger than 60 years of age tend to perform better.74 Several chemotherapeutic agents have been used for relapsed PCNSL. Intraventricular rituximab is an alternative for patients who did not receive it as an initial therapy94 A trial comparing intraventricular rituximab and intraventricular MTX in patients with relapsed PCNSL is ongoing. Primary intracranial germ cell tumors account for 1% of all primary brain tumors in adults and are divided into five types: germinomas (65%), teratomas (18%), embryonic carcinomas (5%), choriocarcinomas (5%), and endodermal sinus tumors (7%).95,96 Germinomas are more often located in the suprasellar region than in the pineal region.97 Germinomas are extremely radiosensitive tumors; patients have 5-year survival rates of more than 90% with radiation therapy alone.98–101 In contrast, tumors containing nongerminomatous malignant elements are less radiosensitive; studies including radiation therapy with or without chemotherapy show a 5-year survival rate of 20 to 76%.102–104 Results of the Third International CNS Germ Cell Tumor Study, designed to demonstrate the efficacy of a chemotherapy-only protocol in avoiding the additional morbidity caused by radiation therapy of germ cell tumors, were recently published.105 Twenty-five patients with newly diagnosed germ cell tumors were treated with one of two risk-tailored chemotherapy regimens. Regimen A consisted of 4 to 6 cycles of carboplatin/etoposide alternating with cyclophosphamide/etoposide for patients who had low-risk localized germinoma with normal CSF and serum tumor markers. Regimen B consisted of 4 to 6 cycles of carboplatin/cyclophosphamide/etoposide for patients who had intermediate-risk germinoma, positivity for human chorionic gonadotropin β(HCG-β) and/or CSF HCG-β below 50 mIU/mL, and high-risk biopsy-proven nonger minomatous malignant elements or elevated serum/CSF α-fetoprotein and/or serum/CSF HCG-β above 50 mIU/mL. The 6-year event-free and overall survival rates were 45.6% and 75.3%, respectively. The study concluded that chemotherapy-alone regimens are less effective than regimens including radiation therapy. The authors recommended radiation therapy, either alone or with chemotherapy, as the standard treatment for pure germinomas and radiation plus chemotherapy for tumors with nongerminomatous malignant elements. Chordomas are very rare tumors that arise from the remnants of the embryonic notochord; they are characterized by slow growth, a long natural history, frequent local recurrences, and an axial skeleton location (sacrum, skull base, and spine). They are considered low-grade malignancies, but they have the capacity to metastasize to distant organs. Chordomas were diagnosed in 10 patients in a series of 1120 sellar masses treated with transsphenoidal surgery.71 Classically, the treatment of skull base chordomas consists of surgery to reduce tumor volume and, if possible, establish a safety margin for irradiation between the tumor and neurovascular structures. This is followed by some form of radiation therapy or stereotactic radiosurgery.106,107 Historically, chemotherapy has not played a role in the treatment of chordomas, and reports of tumor responses to anthracyclines, cisplatin, and alkylating agents have been anecdotal. In rare instances, chordomas can transform into high-grade sarcomas, becoming a potential target for chemotherapy.76 Recently, a strong expression of EGFR and C-met (mesenchymal-epithelial transition factor) was reported in a series of 12 chordomas.108 In addition, platelet-derived growth factor receptor β (PDGFR-β) was overexpressed in a series of 31 chordomas.109 Because chordomas seem to express EGFR and PDGFR, these tumors may be amenable to treatment with currently available targeted therapy. Several cases of the successful use of imatinib and erlotinib, tyrosine kinase inhibitors, have reported.110–112 Meningiomas are one of the more common brain tumors in adults. About 90% of them are World Health Organization (WHO) grade 1 (benign), 5 to 7% are WHO grade 2 (atypical), and only 3% are WHO grade 3 (malignant or anaplastic).113 Historically, the gold standard treatment for meningiomas has been complete surgical resection. Chemotherapy is reserved for more aggressive tumors, recurrent tumors, or those localized in areas of difficult surgical approach; it is also used after radiation therapy when other options have been exhausted. Although chemotherapy is sometimes useful, reliable, effective agents are lacking and remain an area of investigation. Chamberlain reported a series of 14 patients who had malignant meningiomas treated with adjuvant chemotherapy that included cyclophosphamide, Adriamycin, and vincristine (CAV); the patients showed a modest improvement in survival when compared with patients treated with surgery alone.114 In a prospective Phase II study of TMZ in 16 patients with refractory meningiomas, no patient demonstrated PFS at 6 months; therefore, the study was terminated, and the conclusion was that TMZ is ineffective in meningiomas.115 In a study investigating the MGMT promoter methylation status in 36 meningiomas, none of the specimens showed MGMT gene promoter methylation, providing a rationale for TMZ ineffectiveness.116 Twelve patients with recurrent postoperative residual masses or inoperable meningiomas were treated with INF-α and followed with PET. In five patients treated from 9 months to 8 years, INF-α seemed to be an effective oncostatic drug, deserving of further studies.117 Irinotecan (CPT-11), a topoisomerase I inhibitor, demonstrated growth-inhibiting effects in primary meningioma cultures and in malignant cell lines in vitro and in vivo, but the drug proved ineffective in a Phase II study.118,119 Several successful reports of hydroxyurea, an oral ribonucleotide reductase inhibitor against meningiomas, have been published.120–125 Schrell at al, after demonstrating that hydroxyurea inhibits growth of cultured human meningioma cells and meningioma transplants in nude mice by inducing apoptosis,124 reported a successful outcome in four patients who had recurrent and/or unresectable meningiomas treated with hydroxyurea.125 In another study, of 21 patients with recurrent and progressive meningiomas treated with fractionated three-dimensional conformal radiation therapy and concurrent hydroxyurea, disease stabilization was achieved in 14 patients (66%). Progression-free survival rates at 1 and 2 years were 84% and 77% respectively.121 A Phase II study to further evaluate the role of hydroxyurea in meningiomas (SWOG-S9811) has been completed, but the results have not yet been reported. PDGF and its receptor PDGFR are frequently co-expressed in meningiomas. A Phase II study of imatinib mesylate (a PDGFR inhibitor) for recurrent meningiomas accrued 23 patients (13 with benign, five with atypical, and five with malignant meningiomas), of whom 22 were eligible. Overall median PFS was 2 months (0.7–34 months), and the 6-month PFS rate was 29.4%. For benign meningiomas, median PFS was 3 months (1.1–34 months), and the 6-month PFS rate was 45%. For atypical and malignant meningiomas, median PFS was 2 months (0.7–3.7 months), and the 6-month PFS rate was zero. Imatinib was well tolerated but did not show any significant activity against recurrent meningiomas.126 Meningiomas also overexpress EGFR. Based on this observation, a Phase II study with the EGFR inhibitors gefitinib and erlotinib for recurrent meningiomas was conducted. The study accrued 25 patients. Sixteen patients (64%) received gefitinib, and nine patients (36%) received erlotinib. Eight patients (32%) had benign tumors, nine (36%) atypical meningiomas, and eight (32%) malignant meningiomas. No objective imaging responses were seen, but eight patients (32%) maintained stable disease. The study concluded that although well tolerated, neither gefitinib nor erlotinib appears to be effective against recurrent meningiomas.127 Clinical trials of treatments for recurrent meningiomas, including target therapies with bevacizumab (a monocloncal antibody against vascular endothelial growth factor [VEGF]), sorafenib, sunitinib, and vatalanib (targets VEGFR and PDGFR), are under way. Intrasellar plasmacytomas are rare and can mimic nonfunctional pituitary adenomas clinically and radiologically. Plasmacytomas can progress to multiple myeloma, or multiple myeloma can be diagnosed simultaneously.128 Traditionally, plasmacytomas have been treated with surgery and radiation therapy for local or residual disease. Chemotherapy is added for systemic myeloma treatment. Sinnott et al, in a review of 22 cases of intrasellar plasmacytomas reported in the literature, found that radiation therapy was administered to the sellar lesion in 71% of the patients after surgery and systemic chemotherapy was administered to 29% of the patients following the diagnosis of multiple myeloma.128 Patients undergoing chemotherapy for multiple myeloma comprise two groups: those who are candidates for autologous hematopoietic cell transplantation (HCT) and those who are not candidates for HCT. In patients who were not candidates for HCT, three randomized trials demonstrated superior PFS in those treated with melphalan, prednisone, and thalidomide (MPT) versus patients treated with melphalan and prednisone.129–132 Bortezomib, melphalan, and prednisone (VMP) are an alternative to MPT.133,134 In patients who are candidates for HCT, induction chemotherapy with thalidomide and dexamethasone is the standard treatment.135,136 Lenalidomide (a thalidomide analogue) combined with dexamethasone seems to be an alternative and is highly effective and well tolerated.137 Bortezomib with dexamethasone is also a well-tolerated and effective alternative.138 The term hematopoietic cell transplantation indicates transplantation of progenitor (stem) cells from any source (eg, bone marrow, peripheral blood, cord blood), and HCT may be the only treatment with a chance of producing cure. Although not a first-line treatment for most sellar and parasellar tumors, chemotherapy remains a valuable option for selected patients. The histology, grade, and behavior of the tumor will shape the decision to employ chemotherapy and the type of agents used.
 Pituitary Carcinomas
Pituitary Carcinomas
 Temozolomide
Temozolomide
 Craniopharyngiomas
Craniopharyngiomas
 Pituitary Lymphomas
Pituitary Lymphomas
 Germ Cell Tumors
Germ Cell Tumors
 Chordomas
Chordomas
 Meningiomas
Meningiomas
 Plasmacytomas
Plasmacytomas
 Conclusion
Conclusion
References