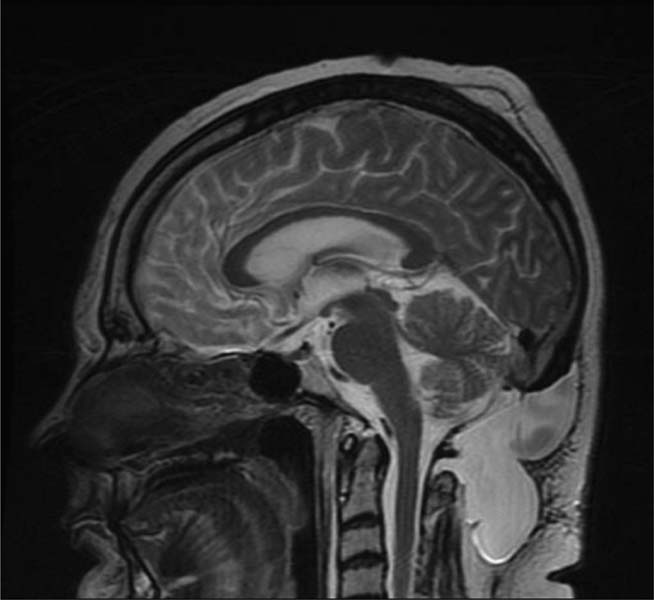
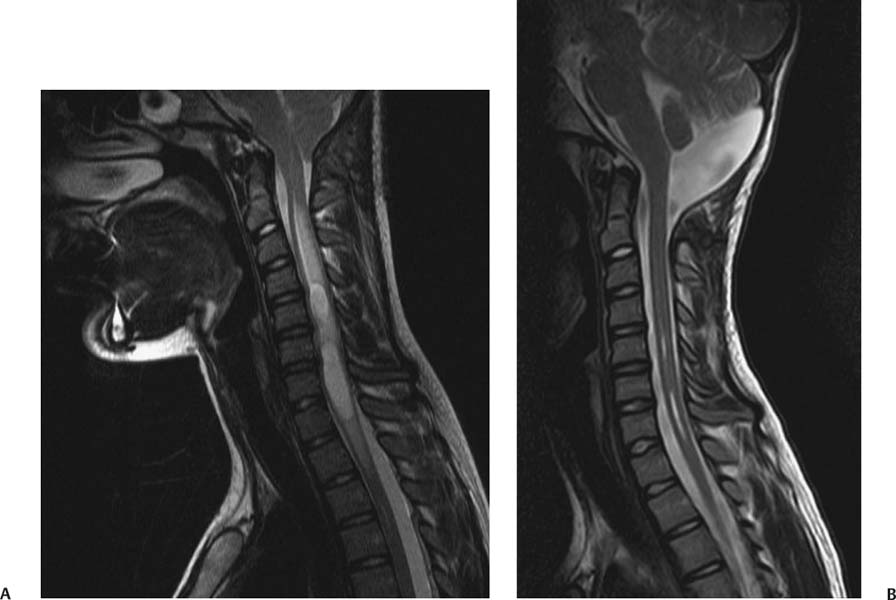
12 Chiari Malformations In this era of prevalent magnetic resonance imaging (MRI), Chiari malformations and syringomyelia are being diagnosed with increasing frequency. Although tonsillar ectopia appears to be a unifying anatomical marker, it is important to recognize that Chiari malformations are heterogeneous conditions with different pathophysiologic mechanisms, which can often cause overlapping symptoms. To treat these patients, each patient’s tonsillar descent must be evaluated in the setting of morphometric analysis of the posterior fossa and stability of the craniovertebral junction. Tonsillar herniation may arise from congenital causes with small posterior cranial fossae (classic Chiari I malformations and craniosynostotic syndromes) and acquired causes with normal posterior cranial fossae (hydrocephalus, Paget disease, posterior fossa tumors, and spinal hypotension). Because of the varied manifestations for Chiari malformations, there have been differences in treatments and surgical outcomes. Therein lies the controversy. Ultimately, successful management depends on appropriate patient selection, tailoring the surgical intervention to treat the underlying anatomical disorder, and complication avoidance. The anatomical factors that need to be assessed are the level of cerebellar tonsillar descent, degree of cervical-medullary compression and foramen magnum impaction, presence of skeletal anomalies (basilar impression, platybasia, odontoid alignment, pannus formation, joint hypermobility, and craniocervical instability), and disturbance of cerebrospinal fluid (CSF) circulation (hydrocephalus or syringomyelia). To evaluate all these variables, we routinely perform an extensive imaging work-up. For each new patient, at least one full neuraxis brain and spine MRI is performed. In selected cases, a gadolinium-enhanced study is performed to exclude the presence of any malignancies. A three-dimensional computed tomography (CT) scan is also obtained, which is particularly useful for revision surgery. Occasionally, this CT will also reveal bony variations, such as an incomplete bifid C1 lamina or C1-occipital assimilation, and aberrant vertebral artery anatomy. Patients develop symptoms related to Chiari malformations from two primary mechanisms: direct brainstem compression and disturbance of CSF flow.1 The most common symptom is the suboccipital headache that may radiate to the vertex, behind the eyes, or to the shoulders and neck. Cranial nerve signs may include impaired gag reflexes, facial sensory loss, and vocal cord paralysis. Ocular, otoneurologic, and cerebellar disturbances are also varied and common. Pediatric patients may demonstrate different clinical manifestations. The youngest patients may present with poor Karnofsky scores related to failure to thrive, because of poor oral intake. Other pediatric patients may have vague behavioral problems before they are diagnosed. Some adolescent patients may present after having had orthopedic repair of scoliosis with unrecognized syringomyelia. Finally, an incidental Chiari malformation may be found after a traumatic event. These patients are first given conservative treatment, which often involves pain management and clinical monitoring of the syrinx if present. After conservative remedies have been exhausted, the three main indicators for surgical interventions are poor Karnofsky score (≤ 70), new-onset or progression of syringomyelia (particularly syrinxes occupying > 75% transverse diameter), and severe neurologic deficit. The goals of Chiari surgery are fourfold: 1. Adequate decompression of the cervicomedullary junction, which includes neural structures and relief of CSF block 2. Creation of a normal-sized cisterna magna and retrocerebellar spaces 3. Establishment of optimal CSF flow between cranial and spinal compartments 4. Stabilization of the craniocervical junction if there is excessive joint hypermobility Since the first posterior fossa decompression procedure performed by Penfield and Coburn in 1938, there has been significant improvement in surgical morbidity and mortality for the treatment of Chiari malformations. The traditional surgical approach includes bony posterior fossa decompression. In surveys sent to international pediatric neurosurgeons (2004)2and American pediatric neurosurgeons (1998),3 suboccipital decompression was considered the standard surgical procedure. The majority of respondents favored routine dural opening at surgery and closure with a pericranial or synthetic patch graft. This technique has been adopted by many surgeons, because it definitively improves the flow of CSF at the level of the foramen magnum. Current surgical debate primarily rests on whether or not to perform a duraplasty. Fig. 12.1 Sagittal magnetic resonance imaging (MRI). Postoperative pseudomeningocele formation from an outside institution requiring reoperation for cerebrospinal fluid leak. The majority of published large case series support the overall benefits of duraplasty in achieving definitive surgical treatment.4–13 The controversy arises from a higher associated morbidity with duraplasty and intradural manipulations. As a result, some surgeons are advocating bony decompression only, as there appears to be a subset of patients who do respond to this intervention.14–17 In effect, the surgeon’s decision is often determined by balancing the risk of a complication (Fig. 12.1) versus the risk of undertreatment, necessitating a return to the operating room (Fig. 12.2). Duraplasty and intradural manipulations have been associated with higher morbidity in certain series. When the arachnoid is opened, there is increased risk for bleeding and adhesion formation, which can lead to arachnoiditis, pseudomeningoceles, hydrocephalus, and persistent syringomyelia. In turn, these conditions may cause persistence in symptoms or new posterior fossa syndrome complaints. The advantage of opening the dura is that it provides the necessary exposure to allow for internal decompression. The effect of chronic severe foramen magnum impaction by the cerebellar tonsils is the formation of arachnoidal adhesions, which may be the primary pathologic focus. The adhesions may be quite pervasive involving the brainstem, posteroinferior cerebellar artery, and spinal cord. Microlysis of the adhesions is an important part of the internal decompression. Additionally, we advocate tonsillar reduction if the obex area is closed and there is no evidence of pulsatile flow of CSF from the fourth ventricle. At the end of the operation, the tonsils are ideally positioned slightly above the level of the putative foramen magnum. Those surgeons who elect not to open the dura may not address the potential significant impact of arachnoidal scarring. Most surgeons have moved away from various intradural techniques, including posterior fossa stenting, catheters, and plugging of the obex, which have not been associated with improved outcomes.18 The one popular exception is tonsillar shrinkage or complete tonsillar amputation. Some have argued that neural tissue should be preserved whenever possible.19 Reduction of the cerebellar tonsils appears to be well tolerated. At the time of surgery, cystic changes are often apparent as a consequence of chronic compression and ischemia.20 There is evidence that the cerebellar tonsils have no neurologic function, and bilateral tonsillectomy is not associated with neurologic deficits. Although the majority of surgeons who perform duraplasties close the dura, there have been some reports, with good outcomes, supporting leaving the dura opened12 or stitched laterally to the muscles.21 According to the authors, it is important to preserve the arachnoid plane. Postoperative complications were related to arachnoidal violations. Limonadi et al reported that a dura-splitting decompression compared with duraplasty can result in reduced operative time, hospital stay, and cost with equivalent early outcome.22 The counterargument is that one return to the operating room for incomplete decompression would significantly tip the cost-effective analysis in favor of duraplasty. Duraplasty material provides another variable for surgeons to choose. Duraplasties can include autologous versus nonautologous graft materials. Autologous grafts may be harvested from fascia lata, ligamentum nuchae, and pericranium. Nonautologous materials include, in decreasing order of use, bovine pericardium, cadaveric dura, and synthetics. These products may be favorable because they decrease operative time. We prefer autologous pericranium because of the decreased incidence of inflammatory reactions and CSF leaks.23,24 Fig. 12.2 (A) Sagittal MRI. (B) Initial treatment from an outside institution with only posterior fossa bony decompression resulted in persistent syrinx and symptoms. Subsequent revision employing expansile duraplasty reveals syrinx change within 3 months. Among these variables, perhaps the central question is what is necessary and sufficient in an operation to achieve our surgical goals. To help answer this, we have adopted the use of the color Doppler ultrasound to guide us intraoperatively. Many of the technical decisions are patient specific. The particular variables in our practice include size of craniectomy, levels of laminectomy, degree of tonsillar reduction, and size of duraplasty. The color Doppler ultra-sound is an important tool because it allows us real-time feedback and intraoperative confirmation of the restoration of CSF flow before we leave the operating room.4 Some surgeons have reported that an intraoperative ultrasound may help in deciding whether to remove bone only or perform a craniectomy with duraplasty.9 In our experience, the most effective procedure with minimal complications has been a tailored osseous decompression of the craniocervical junction, duraplasty employing autologous pericranium, and additional intradural steps (microlysis of arachnoidal adhesions, tonsillar shrinkage) as determined by intraoperative color Doppler ultrasonography. In balancing risks versus benefits for any procedure, it is often useful to review those complications that may lead to additional surgery. There are several pathophysiologic mechanisms for development of problems after surgery for Chiari malformations. First, there may be complications directly related to the index procedure, including transcutaneous CSF leaks, pseudomeningoceles (Fig. 12.1), defective duraplasties, meningoceles, cerebellar prolapse, infection, extensive adhesions, cranial settling, and craniocervical instability. Second, there may have been a failure to achieve the original goals (i.e., persistent tonsillar herniation, persistent impaction of the CSF spaces and neuroanatomical structures at the foramen magnum, and persistent and enlarging syringomyelia cavities). Third, there may have been previously unrecognized pathology not treated at the time of decompression (e.g., hydrocephalus, pseudotumor cerebri, basilar invagination, and cranial settling). These complications provide an impetus for further understanding of this complex disorder. Surgery for Chiari malformations continues to evolve with better understanding of the pathophysiologic mechanisms and improvement in surgical techniques. In pediatric neurosurgery, new intriguing procedures are being introduced, such as tonsillectomy without craniectomy for the management of infantile Chiari I malformation.25 More information will be required before the value of this procedure, or any new procedure, can be evaluated thoroughly. It is likely that future advances in the management of Chiari malformations will come from prospective multicenter clinical trials that provide class II evidence of long-term surgical outcomes. Management of Chiari I malformation must consider the wide variability of the disease. The definitive caudal displacement of tonsils first described by Hans Chiari26 often occurs with multiple associated pathologies, including syringomyelia in 35 to 70%,26–28 scoliosis in 25 to 85%,29,30neurofibromatosis type 1 in 6%,31 Klippel-Feil anomaly in 5%,31 basilar invagination in 4%,31
Decompression with Duraplasty
Diagnosis
Surgical Indications
Surgical Goals
Surgical Approach to Decompression with Duraplasty
Complications and Revision Surgery
Decompression without Duraplasty
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree