Axial MRI brain with evidence of multiple left hemispheric infarcts (arrows) on diffusion-weighted imaging (DWI).
Discussion
This case highlights the critical importance of a detailed history and physical examination in the evaluation of patients with extracranial atherosclerotic carotid arterial disease. In evaluating a patient with an ischemic stroke or transient ischemic attack (TIA), there are a number of important variables. The degree of carotid artery luminal narrowing and the appropriate clinical correlation between the patient’s symptoms and their purported pathology are the most critical factors because the benefit of a carotid artery revascularization procedure is determined by the degree of stenosis, and the symptomatic/asymptomatic status of the patient. For patients with high-grade symptomatic carotid artery stenosis (defined as 70–99% luminal stenosis), the number needed to treat (NNT) is eight procedures to prevent one stroke at 2 years. For symptomatic patients with 50–69% carotid artery stenosis, the NNT is 20. For symptomatic patients with <50% carotid artery stenosis, the NNT is 67. The benefit is much less for asymptomatic patients with >60% carotid artery stenosis – with an NNT ratio of 87 at 2 years [1,2]. Thus, identification of symptomatic status and degree of arterial stenosis has profound implications when determining who should undergo a carotid artery intervention.
Defining symptomatic disease thus determines the relationship between the stenotic vessel and the need for intervention. A patient with a left retinal artery TIA, but right carotid artery stenosis, has asymptomatic carotid artery disease. Furthermore, a patient with moderate-grade carotid artery stenosis who has symptoms of a hemispheric TIA may benefit more from a carotid procedure than a patient with moderate-grade carotid artery stenosis and a retinal TIA. Frequently, patients who present for evaluation may not immediately realize they have experienced a minor ischemic stroke or TIA. Patients referred for evaluation of asymptomatic carotid artery stenosis may, on careful examination, have unrealized sensory deficits that are directly attributable to a small subcortical infarct in the vascular territory of a previously assumed asymptomatic stenotic carotid artery.
The illustrative case above also highlights the importance of complete extracranial and intracranial cervicocerebral arterial imaging in all patients being evaluated for ischemic stroke or TIA. In patients with extracranial carotid artery stenosis, there is little benefit for carotid artery intervention if the distal extracranial or intracranial internal carotid artery (ICA) has a tandem stenosis or occlusion that is more severe than the stenosis of the proximal ICA bifurcation. Knowledge of anatomic variants is also important. For example, the prevalence of a “fetal PCA” ranges from 15% to 32% in healthy subjects, and from 5% to 36% in patients with cerebral infarction [3,4]. In this case, our patient might otherwise have been excluded from a beneficial carotid artery revascularization procedure because of lack of awareness of an important variant of cerebral vascular anatomy.
Tip
The clinical case presentation suggested a retinal TIA. However, brain imaging and imaging of the entire cervicocerebral vasculature is important as one needs to consider the possibility of an occipital lobe TIA or infarct due to vertebrobasilar disease or, alternatively, because of a PCA territory lesion in the context of a posterior cerebral artery (PCA) origin off the carotid artery: a “fetal PCA.”
Case 2. Symptomatic carotid artery disease and a middle cerebral artery (MCA) stroke with concomitant atrial fibrillation: exclusion factors and timing of carotid interventions
Case description
A 63-year-old left-handed woman experienced the sudden onset of a headache. Shortly thereafter, her secretary found her confused in her office with difficulty walking. She was taken to the nearby emergency department where her blood pressure was 170/90 mmHg and her pulse was 80, but irregular. Telemetry confirmed she was in atrial fibrillation (AF).
The general examination suggested bilateral cervical bruits versus a transmitted cardiac murmur. On neurologic examination, she was awake and appeared alert, but was confused and was perseverating. She had no gaze difficulties. There was right arm and leg weakness with preserved antigravity strength. Sensory examination showed decreased pin-prick and temperature sensation on the right side. The National Institutes of Health Stroke Scale (NIHSS) score was 7.
Because she had last been seen “normal” within 2 hours prior to admission, she was given intravenous tissue plasminogen activator (tPA). Within the next 2–3 hours, she had resolution of her symptoms except for some mild speech impairment. Diagnostic evaluation showed a left insular infarct. Magnetic resonance angiogram (MRA) of the head was normal. MRA of the cervical vessels showed high-grade left bifurcation extracranial carotid artery stenosis. This was confirmed by carotid duplex ultrasonography. A TTE showed normal left ventricular ejection fraction (LVEF) and normal valve function with left atrial enlargement (LAE), and mild diastolic dysfunction. No atrial or ventricular thrombi were seen.
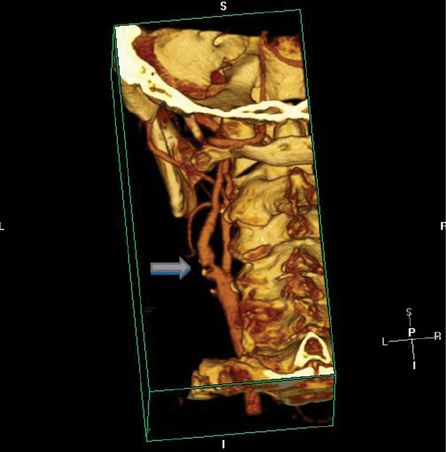
Neurology, cardiology, and surgery consults were requested to determine whether the patient should undergo CEA or should, instead, be started on anticoagulation for AF. Because of AF, the decision was made to first anticoagulate the patient. The patient then underwent inpatient rehabilitation and an outpatient cardiac stress test that was unremarkable. Six weeks later, the patient had a CTA to confirm there was no interval change in the degree of left ICA stenosis (Figure 9.2). The patient had a left CEA, the following day, without complications.
Discussion
Benefits of CEA
The benefit of CEA for stroke risk reduction was established in symptomatic carotid artery disease by two major randomized clinical trials, NASCET (North American Symptomatic Carotid Endarterectomy Trial) and ECST (European Carotid Surgery Trial) [2,5–8]. Subsequently, the Asymptomatic Carotid Artery Study (ACAS) and the Asymptomatic Carotid Surgery Trial (ACST) showed similar, but lesser benefits of CEA in asymptomatic carotid artery disease [2,5,9,10].
The ipsilateral stroke and any perioperative stroke or death aggregate 5-year risk rate in the ACAS study for patients with >60% stenosis was 4.8% for surgery plus medical therapy, and 10.6% for medical therapy alone (absolute risk reduction (ARR) 5.8%; P = 0.04) [9]. Perioperative surgical risk was 2.3%. For ACST, the perioperative 30-day stroke or death rate was 3%, and the 5-year stroke risk (excluding perioperative events and non-stroke mortality) was 4.1% in the surgical arm versus 10.0% in the control arm (P < 0.0001) [10].
For patients in the NASCET study with 50–69% carotid artery stenosis, the ipsilateral stroke and death 5-year aggregate risk rate was 15.7% for surgery plus medical therapy, and 22.2% for medical therapy alone (ARR 6.5%; P = 0.045) [8]. For patients in the NASCET study with <50% carotid artery stenosis, the ipsilateral stroke and death 5-year aggregate risk rate was 14.9% for surgery plus medical therapy, and 18.7% for medical therapy alone – a finding that was not statistically significant (P = 0.16) [8]. The all stroke and death aggregate 2-year risk rate in the NASCET study for patients with >69% stenosis was 14.6% for surgery plus medical therapy and 35.6% for medical therapy alone (ARR 21%) [6]. Perioperative surgical risk was 6%. For symptomatic carotid artery stenosis, but not for asymptomatic carotid artery stenosis, there was a gradient effect: symptomatic patients with a greater degree of carotid artery stenosis accrued more benefit from a carotid intervention (Table 9.1). Similar risk reduction rates were reported in the ECST study with a 3-year major stroke and death aggregate risk of 14.9% in the surgical arm, and 26.5% rate in the medical arm, with a similar gradient in benefit based on severity of carotid artery stenosis [8].
% Carotid stenosis | % Stroke control | % Stroke surgical | Absolute % difference |
|---|---|---|---|
90–99 | 33 | 6 | 27 |
80–89 | 28 | 8 | 20 |
70–79 | 19 | 7 | 12 |
Overall | 25 | 7 | 18 |
The current accepted outcome rates for symptomatic or asymptomatic carotid artery stenosis are based on 1990s data from the North American and European surgical trials. The accepted 30-day risk of perioperative stroke or death is 6% in the context of symptomatic carotid artery disease, and 3% in the context of asymptomatic carotid artery disease [5]. The ACAS study reported a perioperative risk of about 1% related to catheter cervicocerebral angiography, and 2% related to the actual surgical procedure. Arguably, therefore, the postsurgical complication rate, in the absence of preoperative angiography, should probably be held to a standard rate of 2% or less.
Timing of a procedure
Timing of carotid artery revascularization after stroke or TIA is not well defined. There are no strong prospective data, and this continues to be an issue of major clinical concern. In addition, the data are derived from the randomized clinical trials that enrolled patients with TIA or minor disabling stroke with only a few medical comorbidities [11–14]. Thus, decisions about when to intervene are currently based on subjective impressions of various clinical factors.
Retrospective data from observational studies and post-hoc analysis of randomized trials suggest that the reduction of stroke and death following a symptomatic carotid artery event was greatest when the patient was evaluated and treated within 2 weeks of symptom onset [12–14]. In a pooled analysis of the randomized symptomatic carotid endarterectomy clinical trials, the NNT to prevent a subsequent stroke in 5 years was 5 for patients randomized within 2 weeks, versus 125 for patients randomized after 12 weeks [12,13]. There is a slightly greater risk of infarct extension or cerebral hemorrhage with early surgery that presumably can be mitigated with tight control of blood pressure, and other risk factors (i.e., glucose and lipid control) [11]. Among the associated clinical factors governing early versus late surgery are size of infarction on head CT, clinical deficits including level of consciousness, and extent of residual tissue at risk in the ipsilateral carotid artery territory following stroke. Since carotid artery flow is greatest to the MCA under ordinary anatomic conditions, MCA cortical strokes may subsequently have a greater risk of hemorrhagic conversion following an ipsilateral carotid artery procedure, as compared with strokes in other vascular territories supplied by the carotid artery.
In general, patients with TIA or minor clinical stroke, with only small areas of infarction on CT or MRI, are good candidates for early carotid artery intervention within a few days of presentation. For patients who have larger strokes, greater degrees of clinical deficits, or significant medical comorbidities that require stabilization, a more nuanced approach may be appropriate with delays of 4–6 weeks to address medical comorbidities, and define the degree of post-rehabilitation recovery prior to carotid artery intervention.
Tip
While patients with AF were excluded from the original CEA trials, this is not an absolute contraindication to surgery (see also Case 6 about patient selection factors). Ideally, all patients being considered for carotid artery surgery should undergo a formal cardiac evaluation, as there is a high incidence of concomitant CAD in patients with atherosclerotic carotid artery disease.
Case 3. Asymptomatic carotid artery disease
Case description
A 79-year-old right-handed woman presented to her primary physician with complaints of “dizziness.” She described the symptoms as a “woozy” feeling and noted that the dizziness had occurred several times over the past three consecutive days. The dizziness seemed worse with changes in position from sitting to standing but it was not consistently associated with positional changes. She had no prior history of dizziness. She had experienced bilateral visual blurring with the last episode and this was the symptom that prompted her to go to her primary physician. She had no symptoms of chest pain, shortness of breath, or palpitations and her symptoms were not worse with exertion. She had no focal neurologic symptoms.
Medical history included well-controlled arterial hypertension and hyperlipidemia. She was on lisinopril, atorvastatin, and aspirin. She had no prior cardiac history. Her mother died of complications of Alzheimer’s disease, and her father’s cause of death was unknown. She had one sister with a history of heart problems and a brother with a history of CEA for unknown reasons. Surgical history was remarkable for a knee replacement 2 years earlier, and bilateral cataract surgery.
On examination, she had bilateral, soft cervical bruits, the one on the left more prominent. She had an unremarkable cardiac examination. Distal pulses were present in both feet though diminished on the right side compared with the left side. She had a normal mental status examination. Cranial nerve examination was unremarkable. She had normal facial and extremity strength and sensation. Coordination and gait were normal. Muscle stretch reflexes were normoreactive and symmetric. Plantar responses were flexor bilaterally.
The primary physician ordered a head CT that showed scattered areas of subcortical ischemic changes. A carotid artery duplex ultrasound report showed high-grade bilateral carotid artery stenosis (Figure 9.3). The patient was then referred to a stroke specialist for possible ischemic strokes in the context of high-grade asymptomatic carotid artery disease. The neurologist obtained an MRI of the brain that also showed bilateral subcortical ischemic white matter changes without evidence of acute ischemic disease on diffusion-weighted imaging (DWI) sequences. MRA also confirmed high-grade extracranial ICA stenoses at the bifurcations. Complete metabolic panel, lipid profile, and glycated hemoglobin (hemoglobin A1c) were unremarkable.
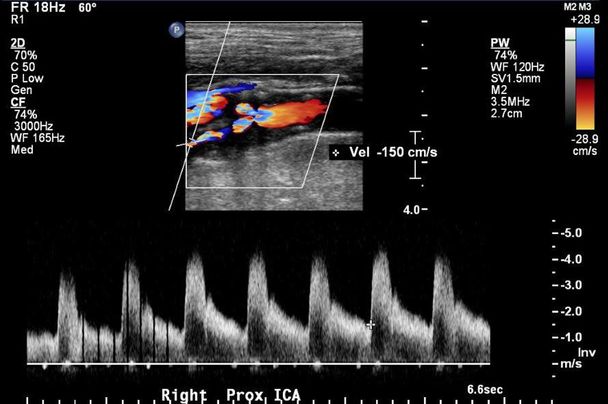
Example of carotid duplex image showing right high-grade carotid artery stenosis.
The stroke neurologist met with the patient to review the imaging findings and the options for carotid artery intervention. The patient elected to undergo a CEA. The surgeon recommended that she undergo a left CEA, with consideration of right CEA at a later date.
Discussion
The benefit of intervention in asymptomatic patients is no greater than for symptomatic patients with <50% carotid artery stenosis for whom carotid interventions typically are not recommended [2,5]. Still, in selected low surgical risk asymptomatic patients with high-grade carotid artery stenosis (arbitrarily >80% to some clinicians, but 60% to one of us, CML, predicated on the ACAS/ACST data) many physicians do offer patients the option of a procedure based on the ACAS clinical trial data (see also discussion in Case 2).
The risk of stroke in people with asymptomatic carotid bruits is relatively low, with an estimated risk of stroke of 1.5% annually [1,2,5,15]. Approximately 4% of the population has an asymptomatic cervical bruit, but a bruit is not a strong predictor of ipsilateral high-grade carotid artery stenosis [15]. Routine screening for asymptomatic carotid artery disease is not recommended because of lack of cost-effectiveness, the small benefit of carotid artery intervention in asymptomatic individuals, and the risk of false-positive or false-negative tests [15–18]. While identification of a cervical bruit may prompt physicians to obtain carotid ultrasound studies, the detection of a bruit does not correlate well with hemodynamically consequential carotid artery stenosis. Even though auscultation of the cervical arteries for bruits is a standard part of the physical examination of adults, detection of a cervical bruit correlates more closely with systemic atherosclerosis than with carotid artery stenosis. For detection of ipsilateral >70% stenosis, the sensitivity of a cervical bruit was only 63% and the specificity was 61% [5,15]. Cervical bruits, however, are a marker of significant CAD; half of persons with a cervical bruit have significant CAD.
Ultrasonography may be appropriate as a screening tool in high-risk populations, but quality and accuracy of vascular laboratories varies widely, and testing should only be performed at certified vascular ultrasound laboratories. Screening might be considered in people aged 65 or older with multiple vascular risk factors or known CAD. However, the benefits of carotid artery disease screening are controversial, and the United States Preventive Services Task Force (USPSTF) report suggested that carotid duplex ultrasonography had significant limits in sensitivity and reliability [18]. The USPSTF report noted that confirmation by angiography for positive tests would expose patients to a 1% risk of stroke and a 30-day perioperative stroke risk of 1.6–3.7% for asymptomatic patients with variations in risk between 1.4% and 6.7% for the combined rate of perioperative stroke and death. In an estimate of “the magnitude of net benefit,” the USPSTF report suggested that 23 strokes would be prevented over 5 years following screening of 100 000 persons. The number needed to screen was 4348 persons to prevent one stroke after 5 years, and 8696 persons screened to prevent one disabling stroke. These rates, however, also presuppose that carotid duplex ultrasonography is accurate enough to identify appropriate persons at risk.
Tip
If there is high-grade bilateral asymptomatic carotid artery stenosis, and there is equal evidence for silent cerebral ischemia on MRI, with no difference in collateral blood flow on either side, the left carotid artery should be preferentially considered for CEA first. If, however, the burden of silent cerebral ischemia is greater on the right side, then one might consider first doing a right CEA preferentially. All patients with a cervical bruit should, however, undergo cardiac stress testing for possible underlying coronary heart disease.
Case 4. Imaging in extracranial carotid artery disease
Case description
A 60-year-old right-handed man presented for consideration of CEA or carotid artery angioplasty and stenting (CAS) after he had a carotid ultrasound that, by report, at an outside vascular laboratory showed 70–95% stenosis of the extracranial right ICA and 50–69% stenosis of the extracranial left ICA with plaque ulceration. There was also high-grade stenosis of the left external carotid artery just distal to the bifurcation. The ultrasound had been done, following a routine physical examination by the patient’s cardiologist, when a cervical bruit was identified on the left side. The patient had no clinical history of strokes or TIAs and no focal neurologic or ophthalmologic symptoms.
Medical history was remarkable for arterial hypertension, hyperlipidemia, and prior history of myocardial infarction 10 years earlier treated with coronary angioplasty and deployment of one coronary artery stent. He was on simvastatin for lipid-lowering therapy, metoprolol, clopidogrel, and aspirin. He was also on pantoprazole for gastrointestinal reflux disease. He had a strong family history of cardiac disease and stroke. He did not smoke cigarettes and drank one glass of red wine daily with meals.
Physical examination showed a blood pressure of 134/84 mmHg and pulse rate of 64 beats per minute and regular. He had a normal cardiac examination with a soft left cervical bruit. Distal extremity pulses were normal. Mental status and cranial nerve examinations were unremarkable. Extremity strength, tone, and coordination, bulk, and gait were normal. Sensory examination was completely normal. There were normal muscle stretch reflexes, and both plantar responses were flexor.
A TTE showed normal LVEF with mild left ventricular diastolic dysfunction and left ventricular hypertrophy. A cardiac stress test 2 years earlier was normal.
MRI of the brain showed a few scattered T2 and FLAIR hyperintensities in the subcortical hemispheric regions bilaterally. MRA of the intracranial circulation showed no intracranial stenosis. MRA of the extracranial circulation showed high-grade left ICA stenosis (Figure 9.4). CT angiogram (CTA) showed moderate- to high-grade bilateral extracranial carotid artery stenosis with significant bifurcation plaque with calcification. At that point, the option for a left CEA was discussed.
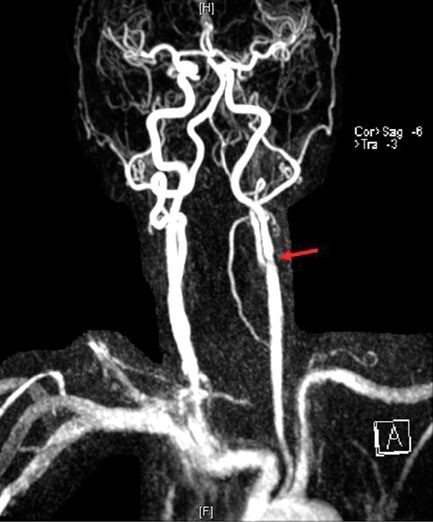
MRA demonstrating extracranial left internal carotid artery stenosis (arrow).
Prior to CEA, however, a conventional catheter cervicocerebral angiogram was obtained. This revealed only moderate-grade stenosis bilaterally with an estimated 60% right ICA stenosis, by NASCET criteria, and 45% left ICA stenosis. A repeat ultrasound was then obtained that confirmed the findings of moderate-grade carotid artery stenosis bilaterally (with lower velocities than seen on the outside carotid artery duplex ultrasound). The patient had a follow-up carotid duplex one year later, with no significant progression in carotid artery disease.
Discussion
Imaging of the extracranial carotid arterial bifurcation is necessary to determine patient eligibility for possible carotid artery revascularization. One of the most common “pitfalls,” however, is reliance solely on a single imaging modality for determination of eligibility for carotid artery revascularization. Many surgeons have resorted to offering patients CEA simply on the basis of one diagnostic carotid ultrasound study. Another common approach for patients with stroke or TIA has been MRI and MRA imaging of the brain, but only carotid duplex ultrasound imaging of the cervical arterial vessels. Both of these approaches for asymptomatic or symptomatic carotid artery disease are woefully inadequate. All patients with stroke or TIA should have brain imaging (preferably MRI of the brain unless there are contraindications), and all screened asymptomatic patients who are then being referred for possible carotid artery revascularization should also have preoperative structural brain imaging to screen for silent cerebral ischemia.
The “gold-standard” by which all other modalities are measured is catheter-guided cervicocerebral contrast angiography [5]. While this modality provides the most accurate imaging measure of stenosis, it is an invasive procedure with associated potential nephrotoxicity and allergic reactions due to contrast dye, as well as potential injury to blood vessels. Cost and the requirements for adequately trained teams also limit the availability of this modality.
The most problematic complication is catheter-related stroke embolism. While the ACAS study reported a catheter-associated complication rate of approximately 1.2%, the rate is probably now much lower at experienced centers.
The most accepted methodology for measuring the degree of carotid artery stenosis is based on NASCET criteria where the length of the residual lumen at the point of maximal carotid artery stenosis is divided by the length of the extracranial carotid artery lumen distal to the stenosis beyond any point of post-stenotic dilatation. Angiography is typically employed when there are conflicting results among the various non-invasive studies, when significant calculation obscures the accuracy of the non-invasive study results, when there are questions about anatomic variations (such as very tortuous carotid artery segments), or in the context of planned CAS. Otherwise, non-invasive imaging has, for the most part, supplanted, invasive catheter angiography in the screening or determination of patient eligibility for a surgical procedure.
Carotid artery duplex ultrasonography is the preferred screening modality for patients with asymptomatic carotid artery disease [15–18]. The threshold of a peak systolic velocity (PSV) >129 centimeters/second (cm/s) has been associated with a sensitivity of 98% and a specificity of 88% for >49% angiographic stenosis [17]. The ultrasonography PSV >199 cm/s has been associated with a sensitivity of 90% and a specificity of 94% for greater than 69% stenosis. However, there were wide measurement criteria variations among laboratories, and the authors implied differences in patients, study design, equipment, techniques, or training [17].
MRA and CTA complement ultrasound in imaging the carotid bifurcation but give additional information about the entire carotid and vertebrobasilar circulation. As such, these modalities are the preferred approach for initial evaluation of patients with possible stroke or TIA symptoms. CTA is limited by costs, problems with post-processing imaging quality, risks of nephrotoxicity from contrast dye, and radiation. MRA is similarly limited by costs, risks of contrast-induced nephrotoxicity, and patient-related factors such as claustrophobia, obesity, presence of MR non-compatible medical devices, and patients not medically able to lie still for MR imaging. Thus, MRA and CTA are not suitable screening tools for asymptomatic carotid artery disease. Nevertheless, in lieu of catheter angiography, MRA or CTA should be obtained to complement carotid ultrasonography in all patients being considered for carotid artery intervention.
CTA may have 100% sensitivity and 63% specificity compared to catheter angiography and may have higher specificity using more advanced scanners or post-processing techniques [5,19]. It has been our clinical experience that in the presence of calcification at the cervical carotid bifurcation, which is very common in this patient group, CTA often overestimates the degree of stenosis. For >69% carotid artery stenosis, one report suggested 100% sensitivity and 100% specificity for CTA, compared with catheter angiography. In that report, MRA had 100% sensitivity and 97% specificity for >69% carotid artery stenosis, compared with catheter angiography [19]. Overall, when done under optimal conditions, MRA has been reported to have a sensitivity of 97–100%, and a specificity of 82–96%, for >50% carotid artery stenosis [5,19]. These data presume that all studies are of high quality. MRA is most useful at ruling out significant carotid artery stenosis, and one benefit of MRA is that it is less subject to artifact problems related to calcifications, as compared with CTA or ultrasonography. However, the pitfalls of MRA are overestimation of the degree of arterial stenosis, and limitations in identifying total versus subcortical carotid artery occlusions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree