Axial cranial computed tomography showing diffuse high density collection in the suprasellar cistern extending bilaterally into the Sylvian fissures consistent with subarachnoid hemorrhage.
Discussion
Subarachnoid hemorrhage (SAH) accounts for only 3% of all strokes and affects nearly 24 000 individuals annually throughout the United States [1]. However, it is the most catastrophic stroke type with a case-fatality rate of 50%. Up to a third of survivors are physically disabled and many more are left with cognitive impairment. The overall incidence reported in most populations is 6–7 per 100 000 person-years and increases with age. The incidence in Japan and Finland is up to 20 per 100 000 person-years [2]. The most common symptom is a sudden and severe (i.e., thunderclap) headache. When a patient presents with a thunderclap headache and impaired consciousness as in the case above, the diagnosis is typically straightforward. However, this is not invariably the case and the headache need not be severe. Other symptoms may predominate (see below). SAH can be traumatic or non-traumatic. Non-traumatic etiologies are subclassified into aneurysmal or non-aneurysmal. Approximately 85% of non-traumatic SAHs occur due to ruptured saccular intracranial aneurysms (aSAH). Of the remaining 15%, non-aneurysmal perimesencephalic hemorrhages account for half, with rarer causes in only 5% [3] (Table 16.1, Figures 16.2–16.4).
Inflammatory arteriopathies: |
Primary angiitis of the CNS Infectious aneurysm Granulomatosis with polyangiitis Polyarteritis nodosa Churg–Strauss syndrome Behçet’s disease |
Noninflammatory arteriopathies: |
Moyamoya disease Intracranial arterial dissection Intracranial vascular malformations Cerebral amyloid angiopathy Reversible cerebral vasoconstriction syndrome |
Cerebral venous sinus thrombosis |
Spinal cord vascular malformations |
Sickle cell disease |
CNS tumors Bleeding diatheses |
Drugs: |
Cocaine |
Antiplatelets, anticoagulants, and fibrinolytics |
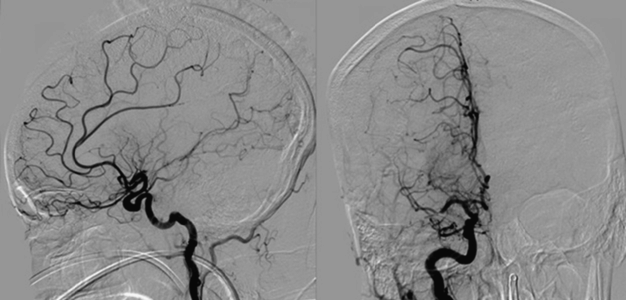
Intracranial aneurysms develop over time. They are not congenital contrary to previous belief. The best estimate of aneurysm frequency for an average adult without risk factors is 3.2% [4]. Modifiable risk factors such as hypertension, smoking, and excessive alcohol intake more than double the lifetime risk of aneurysm formation. Patients with autosomal dominant polycystic kidney disease (ADPKD) or aortic coarctation are also at increased risk for intracranial aneurysms. The chance of harboring an aneurysm in patients with ADPKD is increased nearly sevenfold [4]. The annual risk of aneurysmal rupture varies, and depends on multiple factors including age, aneurysm size and location, sex, race, and ethnicity [5] (Table 16.2). Individuals with a first degree relative in whom an aneurysm rupture occurred carry a lifetime risk of 2–5% for aSAH [6].
Territory | <7 mm | 7–12 mm | 13–24 mm | ≥25 mm |
|---|---|---|---|---|
Anterior circulation (ICA, ACOM, ACA, MCA) | 0% | 2.6% | 14.5% | 40% |
Posterior circulation (PCOM, basilar, PICA) | 2.5% | 14.5% | 18.4% | 50% |
ACOM, anterior communicating artery; PCOM, posterior communicating artery; PICA, posterior inferior cerebellar artery.
Early recognition and diagnosis of aSAH is imperative to reduce overall mortality and improve functional outcomes. Nearly half of patients die from the initial rupture regardless of intervention and the mortality rate for those whose aneurysms rebleed prior to obliteration is even higher. Of surviving patients, 25% incur moderate to severe disability and require long term supportive care. Early diagnosis leading to early intervention reduces short term complications including recurrent bleeding and delayed ischemic neurological deficit (Figure 16.5), improving long term outcomes [7].
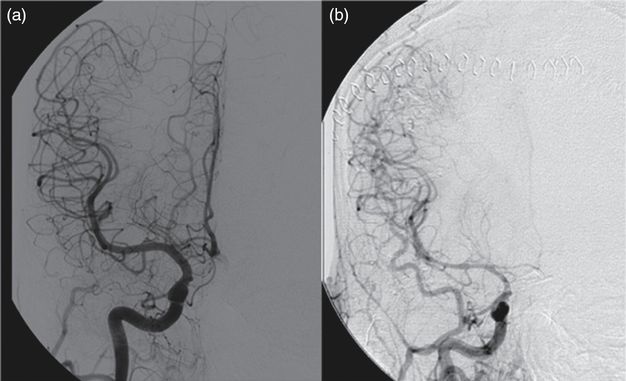
Despite the current widespread availability of neurodiagnostic technology, initial missed diagnosis is common [8]. The impact of missing an early diagnosis of aSAH can be devastating. In a review of patients with aSAH who initially presented to the ED with a headache and normal neurologic exam (Hunt–Hess grade 1 or 2, Table 16.4), misdiagnosis resulted in a fourfold increase in 12-month mortality and worse functional outcomes amongst survivors [8]. The higher mortality and morbidity associated with delayed diagnosis is primarily due to early aneurysmal rebleeding. The recurrent bleeding frequency amongst patients with aSAH is 6.9–17.3% and is highest within the first few hours. Without treatment, 15% of ruptured intracranial aneurysms rupture again within the first 24 hours; 4% on the first day after initial hemorrhage, and 1–2% over the following 2 weeks [9].
Grade | Hunt and Hessa | WFNS scaleb | Fisher scalec |
|---|---|---|---|
0 | Unruptured aneurysm | Unruptured aneurysm | n/a |
1 | Asymptomatic or mild HA | GCS 15 and no motor deficit | No SAH on CCT |
2 | Moderate to severe HA, nuchal rigidity, cranial nerve deficit | GCS 13–14 and no motor deficits | Diffuse SAH <1 mm thick, no clot |
3 | Confusion, lethargy, or mild focal neurologic deficit | GCS 13–14 and motor deficit | Localized clot or layers of blood >1 mm thick |
4 | Stupor and/or hemiparesis | GCS 7–12 ± motor deficit | IVH and ICH without significant SAH |
5 | Coma and/or extensor posturing | GCS 3–6 ± motor deficit | n/a |
WFNS, World Federation of Neurological Surgeons; IVH, intraventricular hemorrhage; ICH, intracerebral hemorrhage; HA, headache; GCS, Glasgow Coma Scale.
a Surgical mortality by Hunt and Hess grade: grade 0–1 = 0–5%, grade 2 = 10%, grade 3 = 10–15%, grade 4 = 60–70%, grade 5 = 70–100%.
b Hospital mortality and WFNS grade: grade 0 = 1%, grade 1 = 5%, grade 2 = 9%, grade 3 = 20%, grade 4 = 33%, grade 5 = 76%.
c Fisher scale grade 3 carries the highest risk of symptomatic vasospasm of the Fisher grades.
Misdiagnosis of SAH may stem from three recurring and correctable factors: (1) lack of appreciation for the clinical spectrum of presenting signs and symptoms, (2) insufficient knowledge of the limitations of imaging, and (3) difficulty interpreting the results of cerebrospinal fluid (CSF) analysis [10]. Knowledge of these factors will assist the physician in correctly identifying patients with SAH and delivering timely treatment.
Tip
This case is a straightforward presentation of aSAH. Following emergent stabilization of airway, breathing, and circulation, immediate attention should be given to management of elevated intracranial pressure (ICP) and rapid identification and treatment of an intracranial aneurysm if present. Hyperventilation, mannitol, and hypertonic saline can be employed. CSF diversion via an external ventricular drain (EVD) may be necessary as acute obstructive hydrocephalus commonly develops. The patient should receive CT angiography (CTA) or conventional catheter-based cerebral digital subtraction angiography (DSA) to assess for an intracranial aneurysm. The latter may also permit therapeutic aneurysm obliteration with endovascular techniques such as detachable platinum coils when feasible based on aneurysm location and configuration.
Pitfalls in the diagnosis of SAH in patients with thunderclap headache, negative CCT scans, and atypical presentations
Case 2
A 38-year-old woman presented to the ED with a sudden onset of the “worst headache of my life” that had been unremitting for the past week. She reported mild neck stiffness but no photophobia, nausea, vomiting, recent illness, or history of previous headaches. She had no known family history of intracranial aneurysms. Her neck was supple and funduscopy was unremarkable. She was alert and oriented with no abnormal sensorimotor signs on neurologic examination. CCT was unremarkable.
Discussion
The estimated yearly frequency of ED visits for headache is 3 million, comprising 2.4% of all visits [11]. The majority of patients with headache seen in the ED have primary headache disorders such as migraine or secondary tension-type headaches [12]. The most characteristic symptom of SAH is thunderclap headache, which occurs in nearly 60% of cases. Thunderclap headaches reach their peak within seconds to minutes, are generally diffuse, and may last up to 2 weeks. In addition to aSAH, thunderclap headaches may also stem from other life-threatening neurologic conditions including meningitis, cerebral venous thrombosis, cervicocerebral artery dissection, pituitary apoplexy, ischemic stroke, colloid cyst of the third ventricle, spontaneous intracranial hypotension, central nervous system vasculitis, and reversible cerebral vasoconstriction syndrome (RCVS). Primary idiopathic thunderclap headache is also possible but requires exclusion of known etiologies. Misdiagnosis of patients that present with thunderclap headache is common and may be due to misinterpretation or misrepresentation of the temporal onset of a primary or secondary headache disorder. In a prospective review, only 10% of patients that presented with sudden headache in general practice were diagnosed with SAH [13]. Clinicians must identify associated red flags so that a prompt clinical diagnosis of a life-threatening etiology can be made. These include sudden onset, age older than 50 years, meningeal signs, altered sensorium, and focal neurologic deficits lasting greater than one hour [14]. Notably, the most important feature of a headache due to SAH is not its presenting severity but rather the suddenness of onset, which is a common misunderstanding.
A sentinel headache in the days to weeks preceding presentation of patients with confirmed SAH has been reported in 1–19% of cases [13,15]. Sentinel headaches are thought to be due to a small rupture and blood leakage, or acute aneurysm expansion. They also have an abrupt onset, although less severe, and should be another red flag for clinicians. The occurrence of a sentinel headache in patients subsequently presenting with aSAH predicts a higher incidence of early recurrent bleeding, overall mortality, and delayed ischemic neurologic deficit [9].
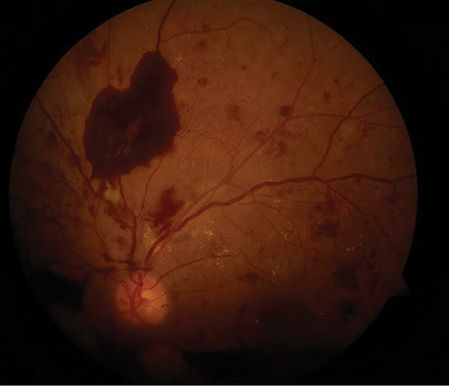
Although up to two-thirds of all patients with SAH have depressed consciousness on admission, there are a number of other clinical manifestations that have been shown to be associated with SAH that can confound diagnosis [16] (Table 16.3). Acute confusion can be misinterpreted as a psychiatric disorder. Neck stiffness due to meningeal inflammation by blood products is common but not invariable. Patients with SAH can present with seizures, and seizure on presentation concomitant with sudden severe headache is a strong indicator of aneurysmal rupture [17]. Focal neurologic deficits such as cranial nerve palsies and hemiparesis can also occur based on anatomic location and volume of hemorrhage. Terson’s syndrome, the presence of associated vitreous hemorrhage, occurs in 13% of patients with SAH [18] (Figure 16.6). Intraocular hemorrhage may also involve the subretinal, retinal, preretinal, or subhyaloid space. Any intraocular hemorrhage in the setting of altered consciousness strongly suggests intracranial hemorrhage with acutely elevated ICP as the cause [19]. Up to 10% of patients with SAH present without headache or focal neurologic deficits. Several aSAH grading systems based on the clinical presentation and CCT findings have been established to estimate surgical mortality, functional outcome, and vasospasm risk (Table 16.4).
Thunderclap headache |
Sentinel headache |
Decreased level of consciousness |
Nausea and vomiting |
Neck stiffness |
Nuchal rigidity |
Papilledema |
Cranial nerve III palsy |
Cranial nerve VI palsy |
Anisocoria |
Seizure |
Terson’s syndrome |
Subretinal hemorrhage |
Retinal hemorrhage |
Pre-retinal hemorrhage |
Subhyaloid hemorrhage |
Low back or leg pain |
Cardiac arrest |
Neuroimaging
Determining a subsequent diagnostic plan for patients with possible SAH and negative CCT scans requires familiarity with the sensitivity and limitations of CCT for detecting SAH. Many tertiary care centers employ third or fourth generation scanners for CCT. With each subsequent generation in development, sensitivity and specificity of CCT for detecting SAH has increased. In the International Cooperative Study of the Timing of Aneurysm Surgery study, the sensitivity of CCT using first and second generation scanners was 92% on the day of aneurysm rupture. It dropped to 86% after only 24 hours and approached 0% at 3 weeks [20]. With more recent technology, sensitivity of CCT for SAH reportedly ranges between 90% and 100%, initially. However, it also diminishes over time [21,22]. The decrease is due to rapid lysis and clearance of red blood cells (RBCs) within the CSF compartment. Thin (3–5 mm) slices are recommended to maximize detection. In alert patients who present within 6 hours of symptom onset, the sensitivity of CCT is as high as 98.5% and decreases thereafter [23]. Therefore, interpretation should be considered based on acquisition time from the ictus. Small volume low-lying SAH in the posterior fossa or above the orbits may also elude detection due to bony artifact from surrounding structures. Magnetic resonance imaging (MRI) has an emerging role in the evaluation of patients with SAH. In the 6 days following SAH, CT and MRI have proven similarly sensitive in the detection of SAH. During the subacute phase (6–30 days) the sensitivity of susceptibility-weighted gradient-recalled echo (GRE) imaging and fluid-attenuated inversion recovery (FLAIR) sequences has been reported to be superior to CT (100% vs. 45%) [24]. Furthermore, MRI may detect other potential etiologies of thunderclap headache that are beyond the resolution capability of CT.
Cerebrospinal fluid analysis
A lumbar puncture (LP) and CSF analysis can be an important diagnostic tool in the evaluation of CT negative patients or those with atypical presentations. Normal CSF is clear and colorless. It has less protein than serum (350 mg/L) and contains no RBCs. Pigment changes that occur within CSF are due to the presence of additional substances, including hemoglobin (red) and bilirubin (yellow). Xanthochromia is a yellow discoloration of the CSF and, when present, is most commonly due to the enzymatic breakdown of hemoglobin. The breakdown process results in the formation of oxyhemoglobin, methemoglobin, and bilirubin. Oxyhemoglobin is released both in vitro and in vivo. However, bilirubin formation requires in-vivo transformation via hemoxygenase (heme to biliverdin) and biliverdin reductase (biliverdin to bilirubin) produced by ependymal cells [25]. Using spectrophotometry, the presence of oxyhemoglobin in CSF is detected rapidly but the formation of bilirubin takes 6–12 hours. This raises the question of whether LP should be delayed when SAH is suspected [25]. There is currently no US guideline addressing this issue. The UK guideline advises waiting 12 hours from symptom onset before performing LP to allow sufficient in-vivo conversion of heme into bilirubin [(26].
Longstanding debate exists as to whether visual inspection or spectrophotometry for CSF fluid pigment analysis is superior. Most hospital laboratories in the US use visual inspection and few possess spectrophotometry. In contrast, spectrophotometry is utilized in nearly all hospitals in the UK [27]. Both techniques involve centrifugation of CSF from the fourth tube collected, followed by examination of the supernatant. Visual inspection compares the CSF supernatant to a tube of water against a white background to assess for xanthochromia (Figure 16.7). Spectrophotometry measures specific amounts of light transmitted at different wavelengths through the CSF and is superior to the photoreceptor cone cells of the human retina for threshold detection and discrimination of pigment wavelengths within the spectral ranges of bilirubin and oxyhemoglobin, particularly at low concentrations.
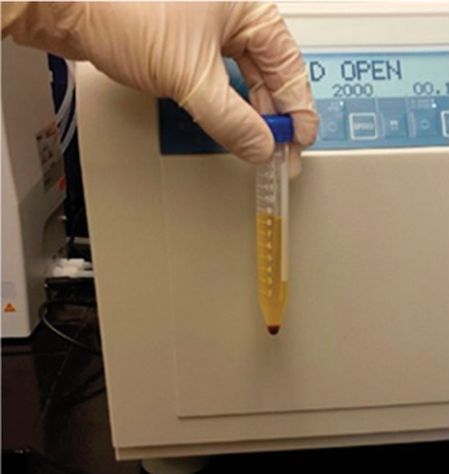
Visual inspection of CSF supernatant for detection of xanthochromia. Courtesy of William Ashley Jr., MD, PhD.
Several studies comparing spectrophotometry to visual inspection for detection of xanthochromia in the CSF of patients diagnosed with SAH have been reported. A review of CSF samples obtained at a neurological hospital in London from 1996 to 2004 revealed that nearly 80% of samples containing substantial amounts of bilirubin detected by spectrophotometry were not perceived visually to have xanthochromia [28]. A study using spectrophotometry as a gold standard showed that visual inspection of purposefully lysed RBCs in samples of CSF had a sensitivity of 27% and specificity of 98% [29]. Another study of 111 patients with CT proven SAH concluded that spectrophotometry performed on CSF specimens collected between 12 hours and 2 weeks of SAH is 100% sensitive for xanthochromia [30]. Despite its high sensitivity, the specificity of spectrophotometry has been questioned by some investigators [30,31]. These authors have raised concerns that the false positive results from universal spectrophotometric analysis could unnecessarily increase DSA utilization leading to the discovery of incidental aneurysms and exposure to unnecessary interventions with periprocedural risks [31]. Moreover, there are other causes of CSF xanthochromia, including ex-vivo lysis of CSF RBCs, that may confound CSF interpretation (Table 16.5).