Fig. 31.1
Chordoma. (a) Coronal T1-weighted pre-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. (c) Sagittal T1-weighted post-gadolinium image. (d) Coronal T2-weighted image. A large, lobular, heterogeneously enhancing mass is centered in the sella, eroding the clivus and displacing the pituitary gland superiorly. The mass is predominantly hyperintense on the T2-weighted image (d). There is invasion of the cavernous sinus bilaterally. A prepontine component is seen abutting the pons
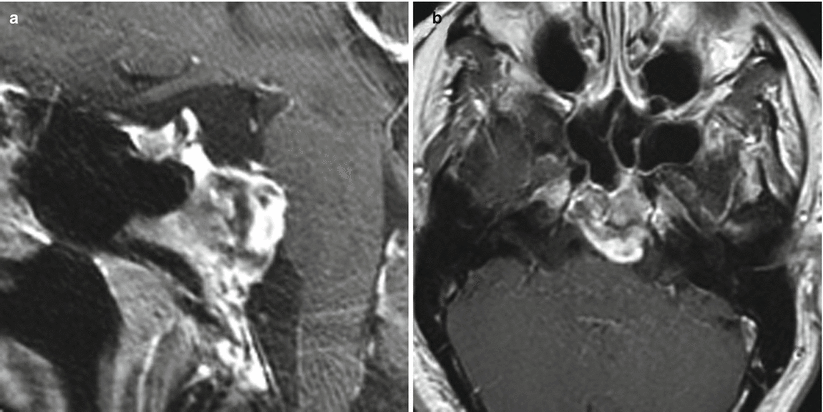
Fig. 31.2
Chordoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Axial T1-weighted post-gadolinium image. There is a lobular, heterogeneously enhancing mass centered in the upper clivus, with an exophytic component extending to the prepontine cistern abutting the pons. The mass appears separate from the sella
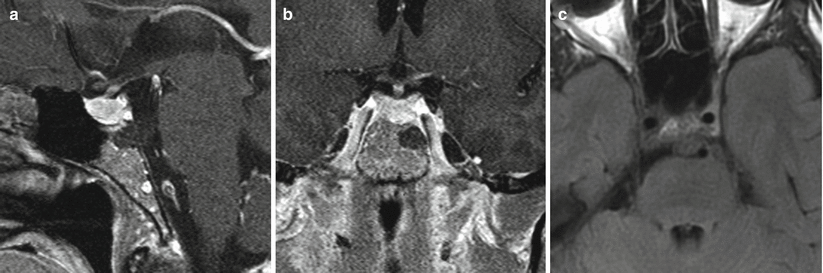
Fig. 31.3
Chordoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. (c) Axial fluid attenuated inversion recovery (FLAIR) image. A small, hypoenhancing mass is located in the left posterior upper clivus, extending to the prepontine cistern. The mass appears separate from the sella
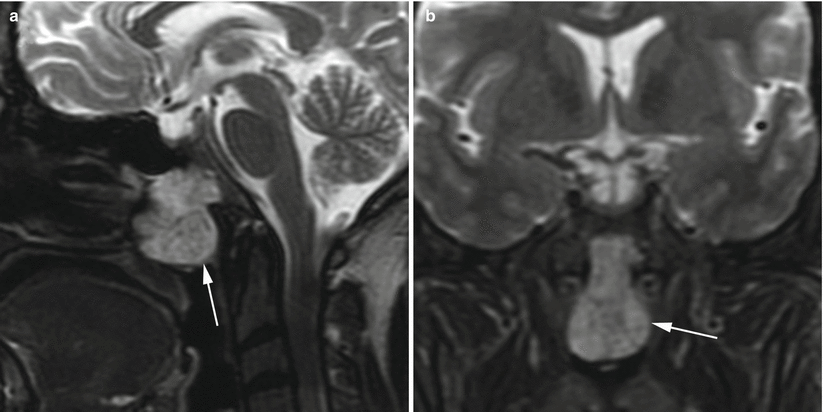
Fig. 31.4
Chordoma. (a) Sagittal T2-weighted image. (b) Coronal T2-weighted image. A lobular, slightly T2 hyperintense mass (arrows) arises from the anterior clivus and extends to the roof of the nasopharynx
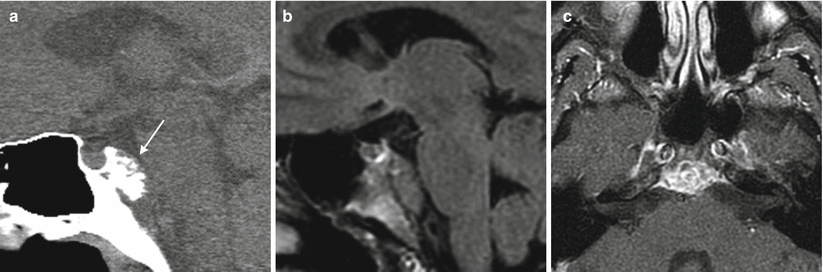
Fig. 31.5
Chondrosarcoma. (a) Sagittal CT image. (b) Sagittal FLAIR image. (c) Axial T1-weighted, fat-saturated post-gadolinium image. A calcified, exophytic mass is centered in the upper posterior clivus (arrow), appearing separate from the sella
31.3 Pathology
Chordomas are low-grade malignant neoplasms derived from the notochord.
Small polygonal cells with eosinophilic cytoplasm and eccentric nuclei are immersed in a mucoid matrix [4]. Vacuolated (“bubble-bearing”), physaliphorous cells are the hallmark finding on histopathological analysis.
The tumor cells are arranged in lobules and can form cords or small nests.
Chordomas typically demonstrate immunoreactivity for S100, vimentin, and epithelial-like markers such as EMA and cytokeratin (Fig. 31.6) [9].
Brachyury is commonly overexpressed in chordomas and can be used to differentiate chordoma from chondrosarcoma and other tumors.
The chondroid subtype of chordoma is characterized by hyalinization and does not appear to be associated with worsened patient outcomes [3, 15].
Microscopic metastatic lesions may be found in up to 40 % of patients on autopsy studies but are grossly identified in 10–20 % [4].
Chondrosarcomas are sarcomatous neoplasms with various degrees of cartilaginous differentiation.
Most chondrosarcomas occur in long bones, but involvement of the base of the skull and sacrum is common.
Chondrosarcomas are characterized by epithelioid-like or spindle cells immersed in a myxoid or cartilaginous matrix similar to that seen in chordomas.
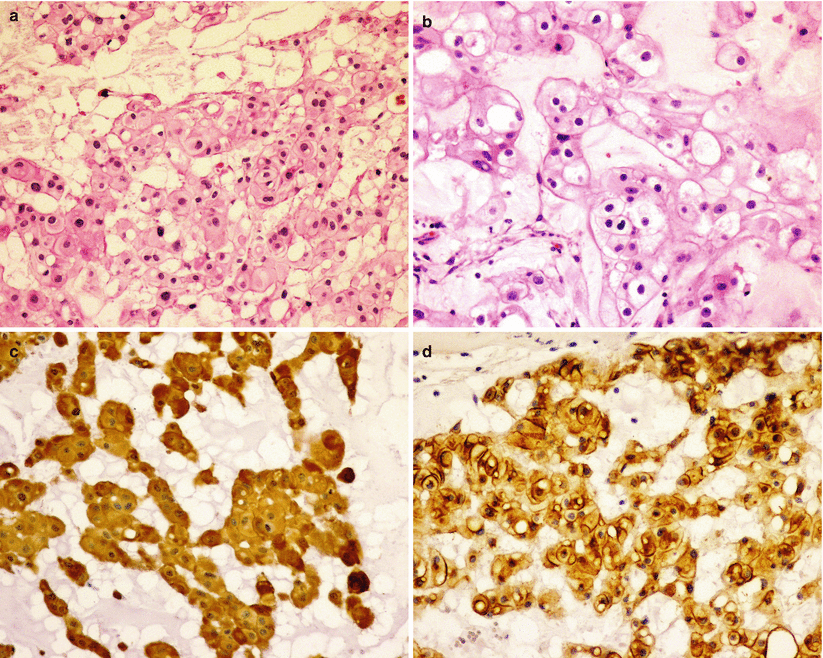
Fig. 31.6
Chordoma. Chordomas are characteristically composed of physaliphorous cells with pale to clear, foamy, or bubbling cytoplasm arranged in cords and trabecules and immersed in a myxoid stroma (a, b). As a derivative of the notochord, chordomas express mesenchymal markers including vimentin and S100 (c), as well epithelial markers like epithelial membrane antigen (EMA) (d) and cytokeratin
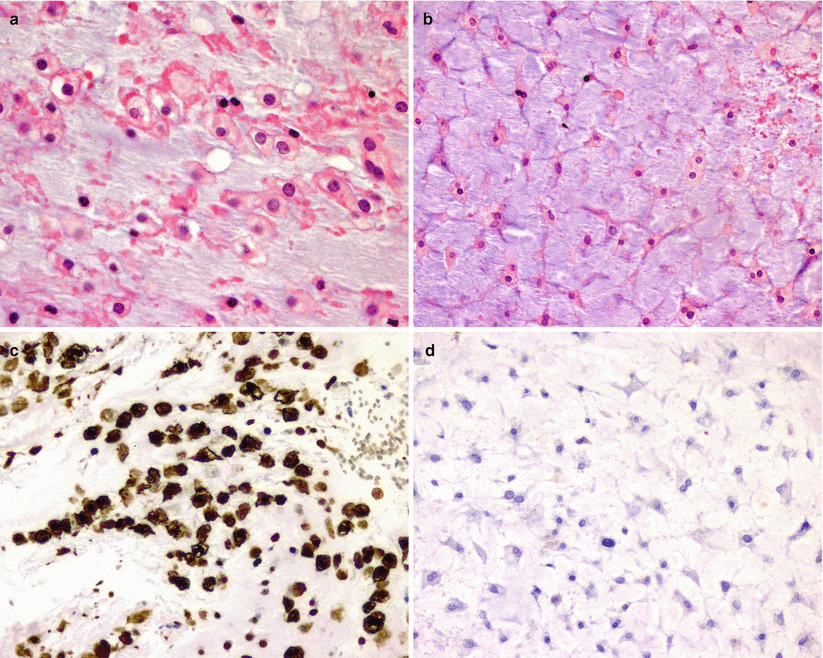
Fig. 31.7
Chondrosarcoma. Chondrosarcoma demonstrates epithelioid cells (a) or stellate-like cells (b) immersed in a cartilaginous stroma, which may be similar to those seen in chordomas. Although chondrosarcomas are immunopositive for S100 protein (c), unlike chordomas, they do not express epithelial markers, including EMA (d)
31.4 Clinical and Surgical Management
Aggressive (if possible, gross total) surgical resection has been advocated for clival chordomas, but resection is frequently limited by extension and invasion of critical neurovascular structures [4, 16].
Chordomas are typically extradural lesions and should therefore be resected via extradural approaches, when feasible [1].
The surgical approach is often dictated by the location and boundaries of the lesion. An extended or combined skull base approach may be preferred to achieve as radical a resection as possible. Far lateral or combined transpetrosal approaches often are indicated to achieve maximal resection of these lesions. In the past, a variety of skull base approaches, including subfrontal, extradural temporopolar, transbasal, transoral, and lateral transcondylar approaches, have been used [1, 11, 17].
Staged procedures are frequently necessary to achieve optimal tumor debulking [11].
Extended transsphenoidal approaches have been used to approach selected chordomas of the clivus [8, 18–20].
In recent years, extended endoscopic techniques have been used to resect selected clival chordomas that do not demonstrate a significant degree of lateral extension or encasement of the vertebrobasilar system [21, 22].
More complex paramedian skull base approaches have been described for resecting the lateral components of these tumors, including the transpterygoid and transmaxillary approaches. The risk of internal carotid artery exposure and injury is increased with these techniques [23].
Extensive skull base reconstruction techniques, such as vascularized, pedicle-based nasoseptal flaps, have been effective in reducing the risk of postoperative cerebrospinal fluid (CSF) leaks [23].

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








