Fig. 18.1
Sagittal T1-weighted MRI (a) showing a large chordoma resulting in severe brain stem compression. The tumor is predominantly hypointense showing some hyperintensity foci that might depict focal hemorrhage. Axial T2-weighted MRI (b) showing heterogeneous signal. The hypointensity foci may represent calcifications, hemorrhage, mucous pool, or intratumoral septa. Bone CT of a chondrosarcoma revealing the characteristic intratumoral calcifications (c)
Chordomas and chondrosarcomas are not differentiated based on exclusively the radiological features, neither in CT nor in MRI [48, 63]. Recently, Yeom et al. have analyzed retrospectively conventional and diffusion-weighted MRI of 19 patients with histologically confirmed chordomas and chondrosarcomas [71]. As expected, conventional imaging features were similar in both tumor types, although poorly differentiated chordomas demonstrated significant low T2 signal intensity. Diffusion-weighted imaging seems to be very promising in distinguishing both tumors because chondrosarcomas were associated with higher ADC (apparent diffusion coefficient) values than classic and poorly differentiated chordomas [71].
18.5 Treatment
Chordomas and chondrosarcomas require a multidisciplinary approach due to the complexity of their management [13, 39], which comprises clinical and radiological observation, biopsy and clinical observation, biopsy and radiotherapy, tumor resection, and surgery followed by radiotherapy [18, 63].
The extent of bone removal has been associated with a long-term disease-free progression and survival [1, 3, 10, 21, 63, 66, 69]. However, the origin of such tumors, which grow from the bone at the skull base intrinsically related to neurovascular structures, frequently precludes radical tumor removal [14, 21, 29, 47, 67]. Besides, radical resection carries a high rate of surgical morbidity, although advocated by some authors [10, 21, 63, 66, 69].
Whereas local recurrence is the most frequent cause of treatment failure [18, 63], treatment is nowadays conducted with a multimodal therapy of surgery plus radiotherapy [18, 39, 67] which offers an improvement in duration of patient survival in comparison to both methods separately [67]. In this regard, prognosis has improved noticeably in the recent years owing to skull base microsurgical development and advances in the field of radiotherapy and neuronavigation [1, 16, 39, 69] (Fig. 18.2). Despite, standard treatment is yet to be defined.

Fig. 18.2
Photograph of the navigation fiducials used for skull base surgery navigation
18.5.1 Surgery
The surgical approaches to chordomas and chondrosarcomas depend principally on the tumor extensions. Tumors may be accessed by anterior, anterolateral, lateral, and posterolateral approaches [54, 63]. Several operative approaches have been used to reach clival area, as follows: transfrontal [54], transbasal [36], unilateral subfrontal [36], extended subfrontal [21, 63, 65], transoral [54, 63], midfacial degloving [6, 7, 36, 63], transsphenoidal [36, 54, 63], frontotemporal [12, 21, 63], frontotemporal with fronto-orbitozygomatic osteotomy [1, 63], frontotemporal subtemporal (extended pterional) [53, 54], subtemporal transpetrous apex [21, 63], petrosal approaches [63], retrosigmoid [54], combined presigmoid-subtemporal (supra- and infratentorial) [52, 53, 58], and far lateral transcondylar [63] or retrocondylar [55], among others.
Endoscopic resection of these tumors has gained much interest recently in literature [19, 20, 31, 68]. Several studies demonstrated successful resection of clival and laterally extended tumors with low complication rates and satisfactory local tumor control in follow-up [19, 20, 31, 68]. In a systematic review, the endoscopic strategy was compared to standard open surgeries in terms of rates of resection and complications [32]. Komotar et al. [32] reviewed 766 patients and concluded that the endoscopic cohort had a significantly higher rate of complete resection, fewer postoperative cranial nerve deficits, fewer rates of meningitis, less mortality, and fewer local recurrences [32]. The open surgical group had an otherwise longer follow-up [32]. As those authors correctly pointed out, endoscopic approaches can be an effective alternative for certain clival tumors. However, it is not performed routinely at our department.
Generally, we use the extended pterional approach for tumors that extend from the upper and middle clivus. Endonasal transsphenoidal approach [23, 41] is preferred in small tumors mainly in the sphenoid sinus or for palliative debulking. For tumors that project ventrally, we perform the midfacial degloving. Whereas tumors at the lower clivus and craniocervical junction, the retrosigmoid approach with or without suprameatal extension [56, 57] and far lateral retrocondylar approach [55] are used, respectively. The combined presigmoid-subtemporal (supra- and infratentorial) approach is reserved for large tumors which extend in both middle and posterior fossae.
18.5.1.1 Frontotemporal Subtemporal (Extended Pterional) Approach
This approach is suitable for tumors that involve structures of the tentorial incisure and cavernous sinus along with posterior fossa extensions to the cerebellopontine angle. A frontotemporal (pterional) craniotomy is developed, in which the bone flap is placed more temporally. The dura is opened basally and reflected superiorly [53, 58]. After releasing the cerebrospinal fluid, brain spatula is inserted to retract the temporal lobe bringing into view the optic nerve, the internal carotid artery, and the anterior structures of the tentorial incisure. Tumors arising laterally are debulked and the hard parts are drilled with a diamond burr. Whereas tumors that extend into the petrous apex, the tentorial margin is opened transversally to improve surgical view together with superior petrosal sinus coagulation and division [53, 58]. Care should be taken with the trochlear nerve that runs along with the free margin of the tentorium. For such reason, the tentorium is opened 1–2 cm behind the trochlear nerve entrance in the cavernous sinus in a medial to lateral fashion [58]. For extradural tumors, the cranial base dura is also opened. Then, further exposure is gained by drilling part of the petrous apex at Kawase’s triangle (Fig. 18.3). Tumor resection is carried out with extensive bone drilling; however, the primary objective is to remove as much as possible without jeopardizing cranial nerves and vessels in order to obviate functional losses. When primary dural closure is not achievable, reconstruction is performed with fascia lata graft or a pedicled temporal muscle flap to cover the defect. Major drawback of this approach is brain retraction.
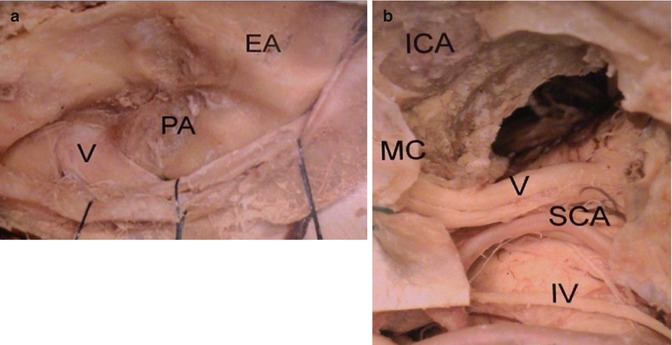

Fig. 18.3
Anatomical dissection of the cranial base detailing the frontotemporal subtemporal (extended pterional) approach to the petrous apex (PA). (a) For extradural arising tumors, the dura mater of the cranial base is opened revealing the petrous apex (PA) and the trigeminal nerve (V), as well as the eminence arcuate (EA). In (b), magnified view of the approach after drilling Kawase’s triangle. Note the superior cerebellar artery joining the trigeminal nerve and the trochlear nerve (IV) at the free tentorial edge. MC Meckel’s cave, SCA superior cerebellar artery, ICA internal carotid artery
18.5.1.2 Endonasal Transsphenoidal Approach
The patient is placed supine with the head slightly extended and tilted toward the surgeon. We use the operating microscope from the beginning of the surgery. By the use of a hand speculum, commonly the right nostril is entered. After identification of the middle turbinate, we coagulate the septal mucosa at the anterior wall of the sphenoid sinus. The mucosa is incised and a right submucosal tunnel is developed. Then, by the help of a high-speed drill, the bone septum is drilled in the way to the contralateral side. The septal mucosa on the left side is identified, and a contralateral submucosal tunnel is created with a fine dissector. Thus, the anterior wall of the sphenoid sinus is entirely uncovered. The hand speculum is substituted by a self-retaining Hardy speculum retracting the mucosa laterally. The sphenoid sinus is entered after drilling the anterior wall of the sphenoid sinus. Sinus mucosa is resected with subsequent tumor identification. Eventually, we use the fluoroscopic C-arm control. The tumor is removed as described above. Lateral extensions of the tumor preclude complete removal. After tumor resection, the mucosa and nasal septum are returned back by opening of a hand speculum in both nostrils. There is no need of suturing the mucosa.
18.5.1.3 Retrosigmoid, Retrosigmoid Intradural Suprameatal, and Far Lateral Retrocondylar Approaches
The retrosigmoid approach is used to treat lesions at the lower clivus or petrous bone that extend posteriorly toward the cerebellopontine angle ultimately displacing the brain stem, whereas the retrosigmoid intradural suprameatal approach (RISA) is preferred for tumors that have a petrous apex component and traverse to the middle fossa via Meckel’s cave (Fig. 18.4). The operative nuances were depicted elsewhere. Tumors arising at the craniocervical junction may be removed through a retrosigmoid approach along with C1 hemilaminectomy [55] (Fig. 18.5). Eventually, the posterior third of the occipital condyle is drilled away. Transcondylar approaches are seldom necessary [58].
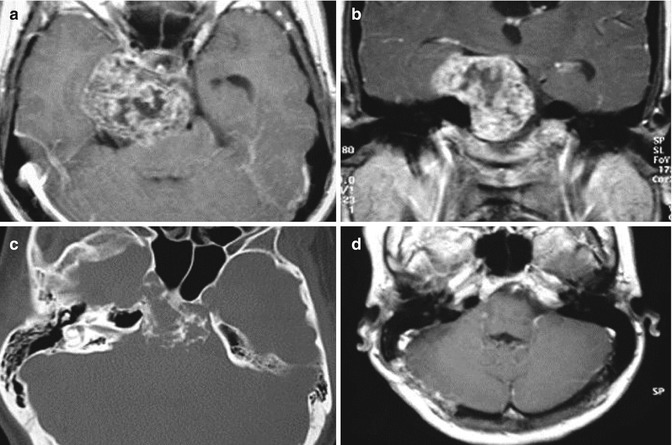
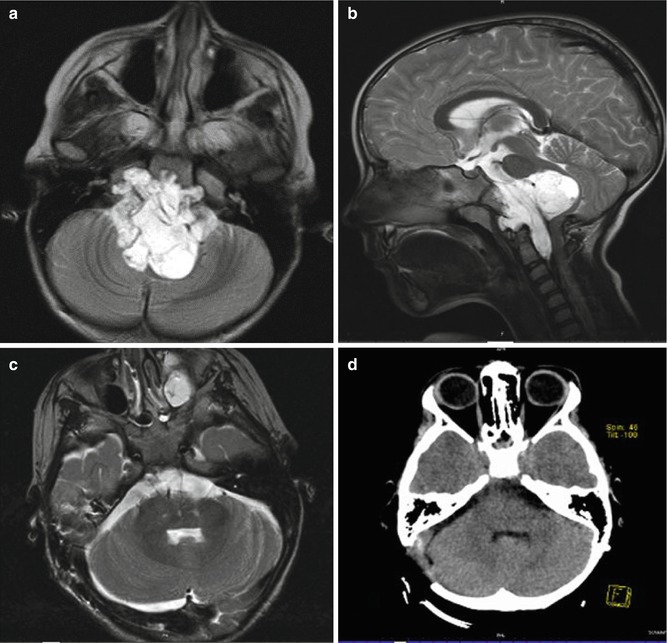

Fig. 18.4
Axial (a) and coronal (b) T1-weighted gadolinium-enhanced MRI shows a chondrosarcoma extending into the middle and posterior fossa. Bone window CT (c) reveals the extensive bone destruction. In (d), postoperative axial T1-weighted gadolinium-enhanced MRI following retrosigmoid intradural suprameatal approach (RISA) showing complete tumor removal

Fig. 18.5
Axial T2-FLAIR (a) and sagittal T2-weighted (b) MRI revealing a large chordoma in a 6-year-old girl. The tumor was completely removed through a retrosigmoid approach together with a far lateral retrocondylar approach on the right side (c, d)
18.5.1.4 Combined Presigmoid-Subtemporal Approach (Supra- and Infratentorial)
The patient is operated on while in the semisitting position or eventually in the park bench position. The head is tilted 30° to the side of the lesion. The preoperative and intraoperative precautions were discussed elsewhere. A skin incision is placed starting 2 cm above and anterior to the ear in a posterior direction along the temporal line (Fig. 18.6). Then, the incision is curved downward extending in a linear fashion posteriorly to the mastoid tip. The temporal muscle is reflected anteriorly and inferiorly. A temporal craniotomy is carried out to the floor of the middle fossa. The transverse sinus is identified and exposed. A suboccipital craniotomy or craniectomy is then performed under direct view of the transverse and sigmoid sinuses. Thereafter, a presigmoid retrolabyrinthine mastoidectomy is accomplished using a high-speed drill in a posterior to anterior and lateral to medial direction (Fig. 18.7). When hearing is preserved, care should be taken not to enter the endolymphatic duct, the posterior semicircular or fallopian canals. The dura incision is created in a T fashion, in which, supratentorially, it is temporo-basal, and infratentorially, it is presigmoid. The temporal lobe is retracted superiorly after exposure and preservation of the vein of Labbé. The superior petrosal sinus is ligated and transected in the way to the free margin of the tentorium. The trochlear nerve is dissected away from the tentorium and preserved. The cerebellum together with the sigmoid sinus and the divided tentorial edge is retracted posteriorly. The tumor is removed as described previously. The dura is closed as usual in a watertight fashion, and the air cells are sealed with fibrin glue and a muscle graft. Disadvantages of this approach consist of its time-consuming and technically demanding procedure, as well as the increased risk of facial palsy, hearing loss, CSF fistula, and vein of Labbé injury.
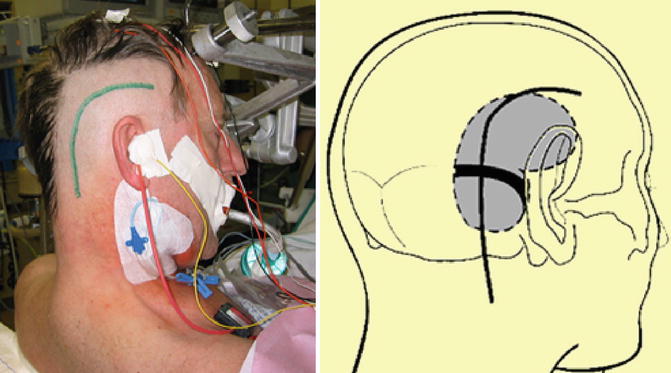
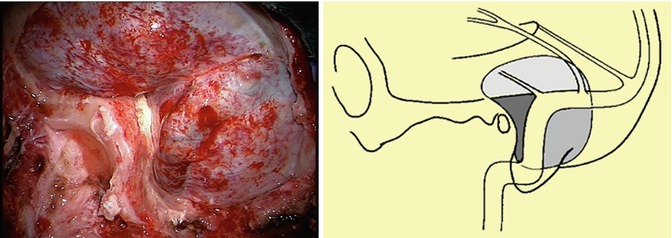

Fig. 18.6
Patient’s surgical positioning (semisitting) for the combined presigmoid-subtemporal approach depicting the skin incision starting 2 cm above and anterior to the ear running posteriorly along the temporal line. Then, the incision bends downward ending posteriorly to the mastoid tip (left). Schematic view of the surgical positioning indicating temporal and suboccipital craniotomy (black dotted line) (right)

Fig. 18.7
Intraoperative view of the combined presigmoid-subtemporal approach right after temporal craniotomy and suboccipital craniectomy (left). Schematic view of the surgical approach showing bone resection and venous relationships (right)
18.5.2 Radiotherapy
18.5.2.1 Conventional Radiotherapy
Conventional radiotherapy is performed for decades in the management of chordomas [9]. Nevertheless, inconsistent results considered chordomas as radioresistant tumors when doses in the range of 45–60 Gy were applied [25, 35, 63]. On the other hand, some studies using escalated doses of radiation (>60 Gy) have showed a dose–response relationship of chordomas and chondrosarcomas [4, 14, 59, 63]. Limitations of its use occur due to radiotolerance of adjacent normal tissues, such as the brain stem or the visual pathways [14, 47, 59]. Actually, controversy still persists concerning the standard dose and radiation protocol in the form that available data of conventional radiotherapy revealed no convincing evidence of symptoms relief or survival after delivery of higher doses of radiation scheme [67]. Owing to the uncertainty of responses associated to the risks of clinical late toxicity, other forms of radiotherapy have been studied [9, 18, 47, 59, 63]. Besides, higher doses of radiation may be delivered with other radiation modalities, such as radiosurgery or proton beam radiotherapy [63]. Therefore, some authors no longer recommend conventional radiotherapy in the management of such tumors [63].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








