Fig. 34.1
Photograph of a paper record made while monitoring intracranial pressure epidurally in a patient with normal-pressure hydrocephalus (NPH). Sequences of symmetrical B-waves, initially described by Lundberg in the 1960s, displaying an amplitude ranging from 10 to 50 mmHg with a frequency of 0.5–3/min have been associated with chronic hydrocephalus. If those waves are recorded for more than 50 % of the time, when assessed during overnight monitoring (48 h), they have been reported to be a good predictor of outcome in a subset of patients. So-called plateau waves with amplitudes of up to 100 mmHg over a period of 5–20 min are rarely observed in chronic hydrocephalus. B-wave activity, however, has been associated with rapid-eye-movement sleep activity, thereby questioning the validity for hydrocephalus diagnosis. It is assumed that B-waves are of vascular nature (i.e., related to changes and oscillations in the systemic blood pressure)
Recently, a single-center study on external lumbar drainage (ELD), done in a larger series of 151 patients with idiopathic NPH, provided comparably high figures for both PPV (90.5 %) and NPV (77.7 %) [31]. In that study, 76 of the 84 patients with a positive drainage response improved with a shunt, and 14 of the 18 patients in whom drainage gave no improvement did not benefit from the shunt, suggesting that a drainage protocol might be most favorable in mimicking a shunt response. Although the risks associated with ELD (e.g., nerve root irritation, infection, and headache) are reported to be low, the true disadvantages (e.g., hospitalization and compliance) remain to be calculated.
In summary, the diagnostic tests reveal evidence of a heterogeneous patient population in adult chronic hydrocephalus, where no constant morphological and physiological element exists. As a consequence, more than one test is routinely applied in the hope of improving the prognostic accuracy; however, the result is a “diagnostic puzzle.” Regardless of the rationale underlying the use of a combination of test procedures in the individual patient, such a concept is questioned by many clinicians, stating that there is clearly a need for a single standardized as well as a practicable diagnostic protocol.
34.4 Neuroimaging and Brain/CSF Metabolism
Basically, ventriculomegaly is indicated by Evan’s index or the frontal horn index, defined as the largest diameter of the frontal horns divided by the largest diameter of the brain at the level of the anterior commissure or at the level of the frontal horns: a value of this parameter above 0.3 or 0.4 is indicative of hydrocephalus. These indices are possibly the only findings obligatory for establishing the diagnosis of hydrocephalus, while all other imaging findings are optional [38], for example, large temporal horns, dilated third ventricle, enlarged perisylvian fissures, or both focal dilatation and obliteration of the cortical sulci (so-called periventricular edema, Fig. 34.2a), increased callosal angle, and aqueductal flow void (Fig. 34.2b).
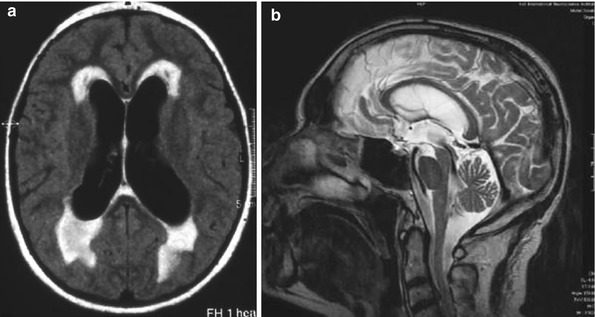

Fig. 34.2
(a) Axial proton density- and T2-weighted turbo spin-echo sequences showing so-called periventricular edema around the frontal as well as the occipital horns, with an irregular-type morphology in NPH. (b) In sagittal T2-weighted images, the flow void at the level of the aqueduct has been associated with an increased aqueductal flow volume, indicating hyperdynamic cerebrospinal fluid (CSF) flow. Studies of the aqueductal stroke volume have suggested measures of 18 ml/min or 42 μl to be indicative of chronic hydrocephalus
Attention should be drawn to periventricular white matter lesions (PWMLs) and deep white matter lesions (DWMLs), as observed in T2-weighted MRI or in fluid-attenuated inversion-recovery sequences (Fig. 34.3). These have been clearly associated with cerebrovascular comorbidity and have been observed in a subgroup of patients with idiopathic NPH [45]. A negative correlation of DWMLs and PWMLs with the outcome in patients was found, although exclusion of an individual patient based on the finding of white matter lesions is not justified, as before shunting, there was no difference in either PWMLs or DWMLs between outcome groups in that study.
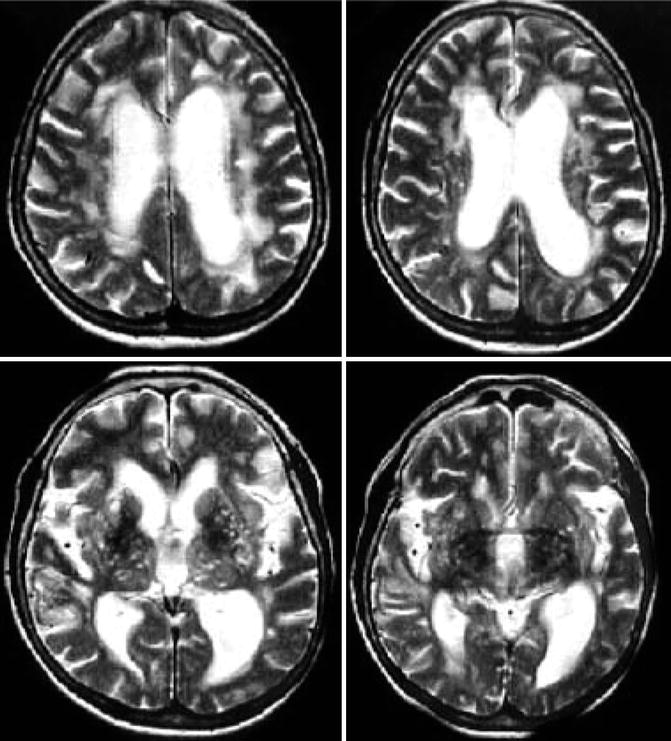

Fig. 34.3
T2-weighted magnetic resonance imaging (MRI) in NPH showing abundant and variable white matter lesions in the periventricular region. They are located in the vicinity of the ventricles, however, distinct from the subependymal zone (upper images). Located in the subcortical regions (lower images), the basal ganglia, they are assigned as deep white matter lesions. These lesions have been attributed to vascular pathology (e.g., chronic subcortical infarctions, normally seen in patients with subcortical arteriosclerotic encephalopathy), systemic hypertension being the most important risk factor
In a comparison of NPH and Binswanger’s disease, it was found that they share, for the major part, MRI changes with regard to white matter lesions, which the authors suggested indicate a common pathophysiological pattern [45]. Mechanisms are explained by an increased pulse pressure in periventricular and subcortical arteriosclerotic vessels, which may cause enlargement of ventricles in the absence of raised intracranial pressure in that subgroup of patients.
Although blood flow and metabolic studies have so far shown a variable correlation with the clinical outcome [35], those methods have been suggested to allow access to the brain disease in hydrocephalus, and studies are promising in this regard.
Using [15O]H2O-positron emission tomography (PET), global cerebral blood flow and cerebrovascular reserve capacity before and after application of 1 g Diamox were quantified before, 1 week after, and 7 months after surgery in 70 patients with idiopathic NPH (Fig. 34.4) [17, 18]. Global blood flow was significantly lower in the “shunt responder.” The predictive accuracy increased to 88 % with the finding of a decreased blood flow, when compared to the accuracy score of 76 % obtained by a combination of dynamic CSF tests. Furthermore, the cerebrovascular reserve capacity was correlated with clinical changes after shunt placement: after 1 week, shunt responders showed considerable improvement (>30 %) in reserve capacity and nonresponders did not, suggesting that neurological improvement after shunting is related to early restoration of the hemodynamic reserve and to improved conditions in the chronic hypoxic environment of the hydrocephalic brain [17].
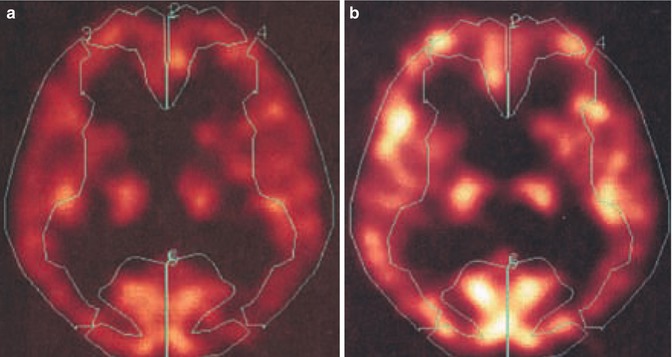

Fig. 34.4
[15O]H2O-positron emission tomography (PET) before (a) and after (b) after intravenous application of 1 g Diamox in a patient with NPH. Diamox (acetazolamide) increases cerebral blood flow through cerebral vessel dilatation. If blood flow increases by more than 30 %, the so-called cerebrovascular reserve capacity is considered intact. [15O]H2O-PET is considered a routine tool in the diagnosis of cerebrovascular diseases. Both blood flow and cerebrovascular reserve capacity in hydrocephalic patients were measured, averaging the measures in the regions of interests according to the vascular territories of the anterior, middle, and posterior cerebral arteries
Using statistical parametric mapping (SPM 99, Wellcome Department of Cognitive Neurology, London, UK) in a series of idiopathic NPH, significant regional decreases in frontal, frontomedial, and temporomedial cortical areas were found, which positively correlated with the clinical impairment using a score based on a formal assessment of both gait and mental function (Fig. 34.5). These areas could have been attributed to the frontal association cortex, to the frontomesial and the supplementary motor cortex, and to the inner and outer limbic cortex after transformation into a standard stereotaxic space (Montreal Neurological Institute default template of SPM 99 for 15O regional cerebral blood flow studies). In addition, after shunting, clinical responders exhibited increases in regional blood flow in supplemental motor areas, while nonresponder did not.
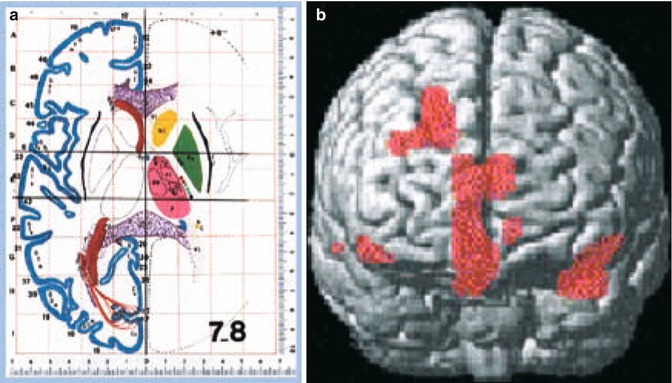

Fig. 34.5
Using statistical parametric mapping (SPM 99, Wellcome Department of Cognitive Neurology, London, UK), regional blood flow was correlated with clinical impairment in 70 patients with idiopathic NPH. On the voxel level, statistical correlations were attributed to Brodmann areas (right) after transformation into anatomic standard (Montreal Neurological Institute) – thresholds for statistical inferences: Z > 3.09, uncorrected p-value on voxel level p < 0.001, extend threshold at least 6 voxel. The more the patient was clinically impaired, areas with diminished blood flow were found in the frontal association cortex, in the frontomesial and the supplementary motor cortex, and to the inner and outer limbic cortex, as plotted in the three-dimensional MRI surface image (right)
Other PET studies using an anatomic region-of-interest analysis on coregistered magnetic resonance done in both secondary and idiopathic NPH demonstrated a reduction in cerebral blood flow in the basal ganglia and the thalamus. This was correlated with the level of function in patients, related to the motor disorder [36].
Disturbances in brain and neuronal metabolism have also been evidenced by 1H- and 31P-MRI spectroscopy investigations in NPH patients [8]: lactate peaks in the periventricular areas and reduced N-acetylaspartate/total creatine ratios in the cortex differed from those in controls and in other forms of dementia. Very recent studies in microdialysis have indicated a situation of postischemic recovery in patients with chronic hydrocephalus [1].
In the light of these observations, the CSF contents of neuronal metabolites and peptides, neurotransmitters, and products reflecting neuronal degeneration (tau, A-beta, neurofilament, and sulfatide) have emphasized the potential advantage of investigating neuronal and brain metabolism and have shown to be effective diagnostic and prognostic markers in adult hydrocephalus [42].
34.5 Pathophysiology of Chronic Hydrocephalus
Whether an increased R out and/or B-wave is linked to the pathophysiology of chronic hydrocephalus has been discussed. Early investigations have shown an association between an elevated R out and a reduced compliance with CSF malabsorption [41]. These parameters have since been associated with chronic hydrocephalus and, it has been suggested, are important diagnostic tools [7].
Biopsy specimens taken at the time of shunt surgery in patients diagnosed with NPH did not show any correlation between an increased R out and arachnoids fibrosis, although arachnoid fibrosis was previously supposed to be the morphological correlate causing CSF malabsorption [2]. There is no normative data on any of these physiological parameters, regardless of the methodology used to estimate R out and/or intracranial compliance; this questions the significance of R out for diagnosing chronic hydrocephalus. It is meanwhile acknowledged that R out naturally increases with age in otherwise healthy individuals [11]. As such, in the subgroup of chronic hydrocephalus of the elderly, much higher cutoff values are likely to be indicative of the disease [5].
In the aforementioned cortical biopsy studies, a high incidence of amyloid plaques and neurofibrillary tangles has been observed in patients with idiopathic NPH [2]; 30–50 % of patients have Alzheimer’s disease on cortical biopsy [16]. It has therefore been suggested that the two diseases may have some common pathophysiology (e.g., diminished clearance of extracellular amyloid-beta peptides, Aβ, and hyperphosphorylated tau proteins, hpTau, from the brain interstitial fluid) [39]. Altered CSF dynamics and decreased expression of Aβ transport proteins at the capillary endothelium may be causal.
These findings were corroborated by an experimental study of kaolin-induced hydrocephalus in aged rats: Aß[1–42] and Aß[1–40] accumulation was evident at 6 and 10 weeks post-hydrocephalus induction [22]. Kaolin-induced blockage of CSF circulation in the basal subarachnoidal space results in hydrocephalus (Fig. 34.6a) and increased resistance to CSF absorption. In the later stages, intracranial pressure normalizes; however, resistance remains above normal values [9]. Increasing amyloid burden at CSF absorption sites, in vessel walls and perivascular spaces, and within the parenchyma was observed (Fig. 34.6b). Low-density lipoprotein-receptor-related protein 1 (LRP-1), the primary capillary endothelial receptor for transport of Aβ out of the brain interstitial fluid, also showed reduced expression [22]. The findings suggest that the age-related impairment of the CSF circulation decreases the clearance or washout of potentially toxic products of brain metabolism from the brain interstitial fluid (e.g., beta amyloid), leading to Alzheimer’s disease pathology in chronic hydrocephalus of the elderly.
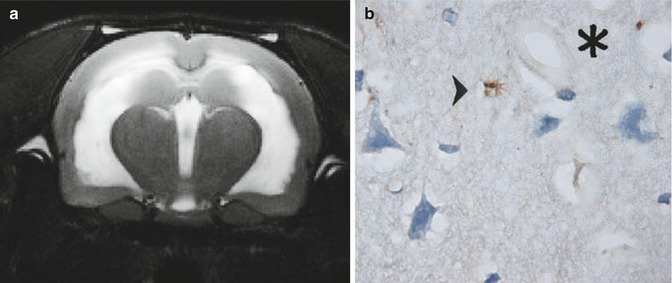

Fig. 34.6
(a) Coronal MRI (4.7 T, Bruker-Spect, Magdeburg, Germany) of kaolin-induced hydrocephalus in a rat. Note the periventricular hyperintensities, obvious at a stage where intracranial pressure normalizes, and resistance to CSF outflow remains above normal levels, indicating a disturbed CSF circulation and turnover. (b) At that stage, using specific immunostaining of anti-A-beta-40 and anti-A-beta-42 proteins in the aged rats with kaolin-induced hydrocephalus, larger “plaque-like” accumulations (arrowhead) in the cortical as well as hippocampal parenchyma were observed; ×1000 microscopic magnification; *cerebral microvessel
Other experimental studies have furthermore supported the role for chronic ischemia causing neuronal injury in vulnerable brain regions and adaptive vascular and neuronal processes [21, 27], playing a more important role in the disease than ventricular enlargement and CSF dynamic parameters.
Experimental studies have contributed to many of the clinical–pathological aspects of chronic hydrocephalus. They allow, however, a more structured insight into the biomechanical as well as the biochemical mechanisms. As a consequence, they have opened the field for adjunct neuroprotective strategies [12].
Since very recently, a proportion of patients in a large idiopathic NPH cohort with large head circumference (HC) have been identified, who presumably have congenital hydrocephalus that has not become clinically apparent until late in life. It is clear that our traditional classification scheme in adult-type hydrocephalus is no longer valid [47].
34.6 Treatment and Outcome
Practically speaking, decision making for shunt surgery results in a variety of different strategies: a strict investigator considers shunting only in patients presenting with the complete triad, marked ventriculomegaly, and other supportive clinical and imaging findings. A liberal investigator shunts nearly everybody, even in the presence of an incomplete triad, comorbid conditions, and/or unexpected clinical and radiological findings. The first investigator may miss an occasionally treatable patient and the second may perform many useless and risky surgical interventions. Neither of the strategies will eventually help to improve the quality of our treatment of NPH but rather may increase the risk of an upcoming “therapeutic nihilism.”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








