CHAPTER 197 Choroid Plexus Tumors
Clinical Background
Choroid plexus tumors are rather infrequent neoplasms, representing less than 1% of all tumors.1–3 However, they are much more frequent in the pediatric population and can represent a sizeable percentage of the tumors in children younger than 1 year of age.4,5 Choroid plexus and its tumors are derived from the specialized neuroepithelial cells in the ventricles. The tumors can recapitulate the characteristics of normal choroid plexus in their structure and appearance and in their function, with the overproduction of cerebrospinal fluid resulting in hydrocephalus. There are also more aggressive forms, with clearly anaplastic features that more closely resemble carcinomas. Significant glial characteristics can also be expressed.
Choroid plexus tumors must be included in the differential diagnosis of the young child or adult presenting with an intraventricular mass. Surgical resection has become an essential part of the treatment of these lesions, with the specific approach based on the tumor location and characteristics. Although the outcome is a function of the tumor type and required treatments, cures and long-term survival are quite possible. See Case 197-1![]() for an illustrative example of evaluation of an intraventricular mass in a 1-year-old child.
for an illustrative example of evaluation of an intraventricular mass in a 1-year-old child.
Case 197-1
Case provided by Dr. Frederick A. Boop, LeBonheur Children’s Hospital and St. Jude Children’s Cancer Center Research Hospital.
One-Year-Old with an Intraventricular Mass
Imaging
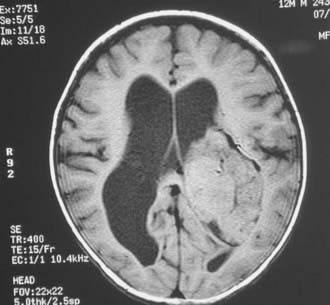
Magnetic resonance imaging clearly showed generalized hydrocephalus and a left intraventricular mass at the atrium consistent with a choroid plexus tumor (Fig. 197-E1). The mass appears to be completely within the left lateral ventricle, with no obvious brain edema, nor any invasion of the parenchyma. Several flow voids are seen within the tumor suggesting large vessels.
The tumor enhances brightly with gadolinium, showing a structure along the superior and lateral margins of the mass that resembles normal choroid plexus. However, more medially, there are definite structural differences (Fig. 197-E2). These differences are seen much better on the T2-weighted images (Fig. 197-E3). In addition, there is a small area of increased signal in the lateral wall of the ventricle that is consistent with brain invasion.
History
Guerard in 1832 provided the first description of a choroid plexus tumor found at autopsy of a 3-year-old girl.6 The first surgical resection was reported in 1906, with the first long-term survival in an adult in 1919.7,8 Until the 1930s, a number of reports focused on the rarity of these lesions and their association with hydrocephalus.8–17 Van Wagenen17 reported excision and good survival of a lateral ventricle tumor in a 3-month-old child in 1930. Dandy18 reported his transcallosal approach to the third ventricle to remove a choroid plexus tumor in a 14-year-old girl. Masson19 reported a transfrontal approach to the third ventricle to successfully remove a third ventricular papilloma in 1934.
Presentation
Incidence
Choroid plexus tumors may occur during any stage of life and thus represent only 0.5% to 0.6% of all intracranial tumors.2,3,20 They are clearly more frequent in the pediatric age range, representing up to 2.9% of all pediatric brain tumors, but account for between 10% and 20% of tumors in infants younger than 1 year.4,20,21 Considering only choroid plexus tumors, 70% are found in children and 50% in children younger than 2 years.5,21–26 Congenital examples have been reported, including tumors detected on prenatal ultrasound.20,27–31 There does not appear to be clear evidence of a sex predilection, with a reported male-to-female ratio of 1.2 : 1.32 Some reports have noted a slight male predominance, whereas others have seen an equal distribution.1,21,25,33–35
The malignant forms (choroid plexus carcinoma) account for 15% to 20% of choroid plexus tumors, but 80% of these malignant tumors are found in children.24,36–39 Although these tumors tend to have a shorter duration of symptoms, there does not appear to be a difference from the benign tumors with respect to age at presentation, sex, type of symptoms, or location of the tumor. The carcinomas have a marked propensity to metastasize.40 Metastasis is the exception for the benign forms, but the satellite lesion retains the benign histology.
Symptoms
The most common symptoms are associated with increased intracranial pressure (ICP).20,22,26,35 The increased ICP may result directly from the large tumor size or from the tumor location obstructing the outflow of cerebrospinal fluid (CSF) and the development of obstructive hydrocephalus. Interestingly, not all choroid plexus papillomas produce hydrocephalus. In younger children, the signs of increased ICP include the sometimes subtle observations of increased head circumference or split sutures before the more obvious signs of vomiting and lethargy. In adult patients, the symptoms can be ataxia, nausea, and vomiting.
In many cases, there is generalized hydrocephalus with ventricular enlargement, not only from obstruction but also possibly from an overproduction of CSF, with up to 800 mL per hour reported in one case.22,23,26,41–47 The overproduction of CSF is also supported by electron microscopic morphologic findings consistent with CSF production, with the frequent resolution of hydrocephalus after tumor removal, and with a report of ascites after shunting but before tumor resection.48 However, increased rate of CSF production has been actually shown in only a few patients.42,43,46,47
Alternative causes for hydrocephalus have been suggested, including the effects of subtle hemorrhage or ventriculitis occurring before any surgery. There is a failure to resolve hydrocephalus after tumor removal in up to 50% of patients, and this could be due to operation-induced hemorrhage or inflammation.21,26,35,46,49,50 It is likely that there are multiple factors of CSF production and absorption that affect the occurrence and resolution of hydrocephalus.
Some patients will manifest focal neurological signs as a result of hemorrhage or focal invasion. For example, tumors located in the fourth ventricle can present with brainstem and lower cranial nerve symptoms related to direct brainstem or cerebellar compression. Patients with tumors in the third ventricle have been reported with various endocrine disturbances, the bobble-head doll phenomenon, and diencephalic dysfunction.16,51–53
Location
Choroid plexus tumors are generally found where the choroid plexus is located—the lateral ventricle in 40% to 50%, third ventricle in 5% to 10%, fourth ventricle in about 40%, and more than one ventricle in about 5%.17,21,25,26,37,40,48,54–57 Primary extraventricular locations have been rarely described, including the cerebellopontine angle, cerebellomedullary cistern, suprasellar cistern, foramen magnum, and spinal subarachnoid space.58–61
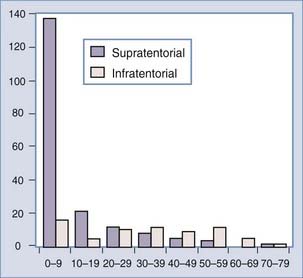
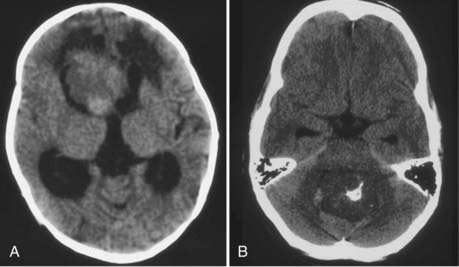
There is a correlation of ventricular location with age, however, that is without an obvious pathophysiologic cause. The lateral ventricular location is most common and the fourth ventricular location least common in children4,20,23,32,56,58,62,63 (Fig. 197-1). In a meta-analysis, the median ages at diagnosis were 1.5 years, 1.5 years, 22.5 years, and 35.5 years for patients with lateral, third, and fourth ventricle and cerebellopontine angle tumors, respectively.32 Bilateral tumors have been reported, but the sheer size of some tumors precludes accurately defining the multifocal origin versus direct extension.23,48,55
The lateral ventricle tumors are typically located at the atrium, although they can be found more anteriorly near the foramen of Monro and in the temporal horn. These tumors are usually very large at presentation, often greater than 4 to 6 cm.21 There may be a predilection for lateral ventricle tumors to occur on the left side, but this is an inconsistent finding. The fourth ventricle tumors are usually in the midline. There can be extension into and through the lateral recesses of the ventricle.32
There are rare reports of metastatic masses derived from the benign forms of choroid plexus tumors. The metastases retain the same pathologic structure and are assumed to be “drop” lesions, disseminating through the CSF.64 It is possible that this group of choroid plexus papillomas showing metastases is a unique and more aggressive variant called an atypical choroid plexus papilloma. There has been one case of pulmonary metastasis.65 However, metastases are a notable feature in the malignant forms of choroid tumors.
The blood supply to the intraventricular tumors is the same as for normal choroid plexus in that ventricle. The principal arterial supply to the choroid of the lateral and third ventricles comes from the anterior and posterior choroidal arteries.66,67 The anterior choroidal artery comes off the internal carotid artery, courses through the choroidal fissure, and supplies the choroid and tumors primarily in the atrium and temporal horn. The posterior choroidal arteries arise from the posterior cerebral artery. The lateral posterior choroidal artery enters the ventricle near the crus of the fornix and supplies the choroidal tissues in the temporal horn, atrium, and body of the lateral ventricle. The medial posterior choroidal artery has a variable supply to the lateral ventricle through the choroidal fissure and foramen of Monro, but it does supply the choroid in the roof of the third ventricle. Thus, tumors of the third ventricle and some in the lateral ventricle can be supplied by branches of this vessel. Because of the significant anastomosis and overlap in arterial supply, both the anterior and posterior choroidal arteries can contribute feeders to these intraventricular tumors.66,67 The choroid of the fourth ventricle is supplied by branches from both the posterior inferior cerebellar and the superior cerebellar arteries.
Diagnosis
Imaging
The evolution of imaging for brain lesions has also affected the diagnosis of choroid plexus lesions. Plain radiography, while done infrequently in the modern era, can show nonspecific calcification within the tumor and nonspecific signs of increased intracranial pressure such as split sutures.1,55,68,69 In the past, pneumoencephalography and ventriculograms were used to indirectly demonstrate these tumors but with significant morbidity and mortality.50 These studies have been replaced with direct imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) usually obtained for the initial evaluation of symptoms in these patients.
Angiography used to be routinely performed to demonstrate the vascular supply of the tumor. The lateral ventricular tumors would consistently show enlarged anterior or lateral posterior choroidal arteries. The third ventricular tumors would be shown supplied by the medial posterior choroidal arteries. The tumors would have an irregular blush. Raimondi and Gutierrez50 provide excellent examples of these angiographic findings. However, currently, angiography is rarely indicated for diagnostic purposes; the vascular supply to these tumors both is consistent and can be effectively demonstrated on MRI.
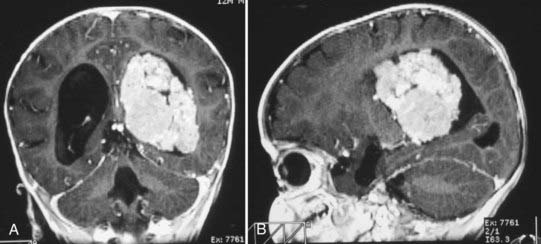
CT will show a variable density to these tumors, ranging from isodense to hyperdense relative to the brain.22,26 Calcification occurs in about 10%26 (Fig. 197-2). The tumor is usually well demarcated from the brain tissue and has rather dramatic enhancement.26 Areas of cystic degeneration can suggest the more malignant choroid plexus carcinoma.22
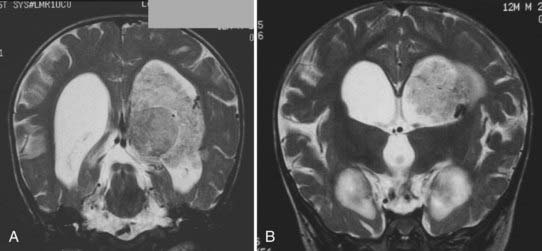
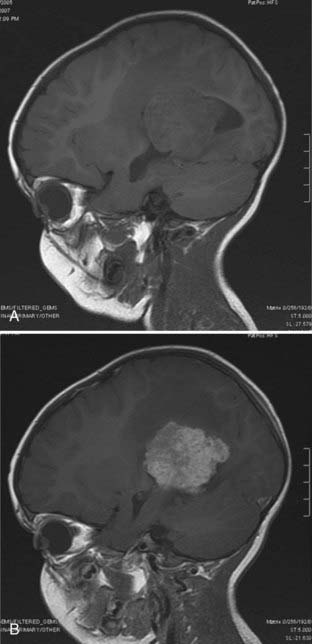
MRI will provide excellent anatomic delineation of the tumor and surrounding brain. The choroid plexus papilloma appears lobulated and separate from the surrounding brain tissue (Fig. 197-3). They are usually isointense to brain on T1-weighted imaging and enhance uniformly. The T2-weighted images show an intermediate to high signal intensity, and the serpentine vascular supply and drainage can be easily seen as flow voids.40,70
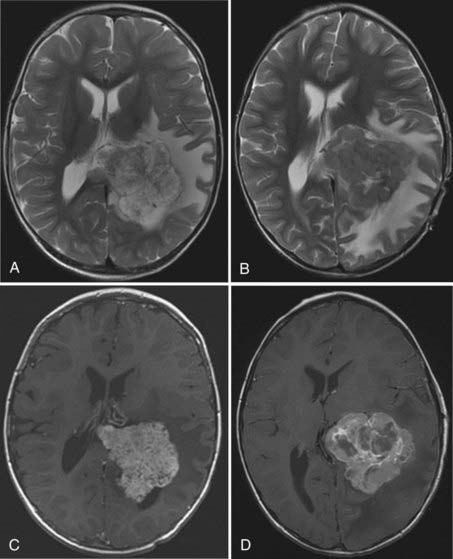
The distinction between papillomas and carcinomas, however, is not always clear-cut. Some papillomas demonstrate adjacent cerebral edema and invasion, whereas some carcinomas do not (Fig. 197-4). Choroid plexus carcinomas often have lost the lobulated appearance and have invasion of the parenchyma with associated vasogenic edema.40,70 There is frequently significant hemosiderin signal change and extensive enhancement with administration of gadolinium-DTPA, although with more variability than seen in papillomas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree