Biomarkers
Specificity
CgA, CgB, pancreastatin
H
PP, NSE, neurokinin, neurotensin
I
HCG-α, HCG-β
L
The chromogranin family and pancreatic polypeptide, neuron-specific enolase (NSE) and alpha HCG constitute general markers for diagnosis and follow-up of patients with NETs.
7.1.1.1 Chromogranins (Table 7.2)
Table 7.2
The chromogranin family
Chromogranin A (CgA) |
Chromogranin B (CgB) |
Secretogranin II (CgC) |
Secretogranin III (1B1075) |
Secretogranin IV (HISL-19) |
Secretogranin V (7B2) |
Secretogranin VI (NESP55) |
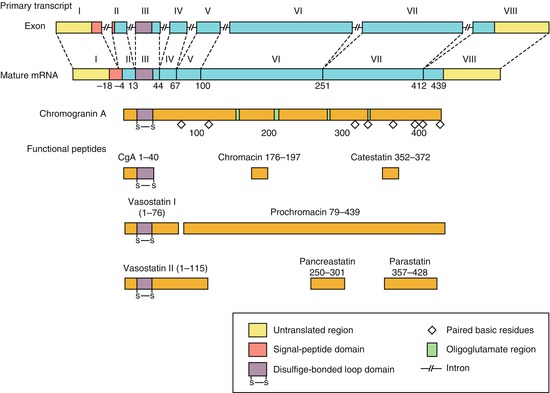
The granins constitute a whole family of glycoproteins of which CgA and CgB are the most clinically interesting. These proteins are 27–100 kDa in size and contain 10% acidic (glutamic or aspartic acid) residues, as well as multiple single and dibasic amino acid residues [9–11]. All of the granins are found as major components of the soluble core of dense-core secretory granules in NE cells and are secreted from these cells in a physiologically regulated manner. The granins are major constituents of large dense-core secretory vesicles and are co-secreted with peptide hormones and mines [9–11]. Granins are found in NE cells throughout the body but also located in the neuronal cells in the central and peripheral nerve system [12]. In adrenal chromaffin cells, CgA and CgB are present in about equal amount, but in thyroid cells and entero-chromaffin cells (EC cells) in the stomach contain mostly CgA and very little CgB [9–11]. CgA, a 439-amino acid glycoprotein, has multiple pairs of basic amino acids distributed along its length, these being more abundant in the carboxyl terminal part of the molecule (Fig. 7.1) [9–11]. CgB has a similar chemical structure being glycoprotein but otherwise different from CgA [13, 14]. The precise function of CgA remains unknown but is thought to be involved in packaging and processing of neuropeptide precursor and peptide hormones [10, 11]. It may also play a role in the organisation of the secretory granule matrix. Moreover, CgA has diverse physiologic interaction. CgA or its splice products are inhibitors of catecholamine, insulin and leptin, having a role in carbohydrate and lipid metabolism; moreover, it inhibits parathyroid hormone secretion. It is also known that CgA increases glucagon and amylase release [15]. In addition to its effect on endocrine organs, CgA also regulates reproductive functions and has a role also in the regulation of cardiovascular functions. CgA elevation has been reported in essential hypertension and in chronic heart failure correlating with the grade of hypertension and cardiac dysfunction, respectively. A role of CgA in the regulation of inflammatory response has been described. CgA is found elevated in various inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus, chronic obstructive pulmonary disease as well as inflammatory bowel disease. CgA in these conditions positively correlates with inflammatory markers such as C-reactive protein and procalcitonin. It is suggested that CgA participates in a negative feedback that limits the activation of endothelial cells [16, 17].
Tumours of neuroendocrine origin usually present with increased plasma or serum levels of CgA and sometimes also CgB. Plasma CgA levels may be elevated in a variety of NETs including pheochromocytomas, paragangliomas, pNETs, small intestinal NETs, medullary thyroid carcinomas, parathyroid and pituitary adenomas and also in a proportion of patients with small-cell lung cancer [4, 8, 14, 18–21]. The highest CgA levels have been found in patients with metastatic small intestinal NETs (carcinoid) and also some pNETs. Both tumour burden and the secretory activity reflect circulating CgA levels. The sensitivity and specificity vary between 60 and 100 % and 70 and 100 %, respectively, for different types of NETs, the highest values being for small intestinal NETs [7, 14, 21–24]. CgA has been shown to be an independent prognostic factor for small intestinal NETs, because it correlates not only with the tumour burden, but also with the biological activity in the tumours (Fig. 7.2). Most functioning and non-functioning NETs present increased circulating levels of CgA. It is also noticed that CgA is more frequently elevated in well-differentiated tumours (G1, G2), compared to poorly differentiated tumours, suggesting a loss of CgA expression in poorly differentiated neuroendocrine carcinomas [25] (Fig. 7.3). Effective treatment is often associated with decrease in CgA values, and the CgA correlates with tumour burden and recurrence [4, 6, 26]. However, in patients treated with somatostatin analogues, there is no correlation between circulating CgA levels and tumour mass [21, 27]. The reason for this is that somatostatin analogues are able to block the production and release of CgA in addition to also affecting tumour burden. Recent data are indicating that early response in CgA might indicate an antitumour effect by treatment with targeted agents (everolimus, mTOR inhibitor) [28, 29].

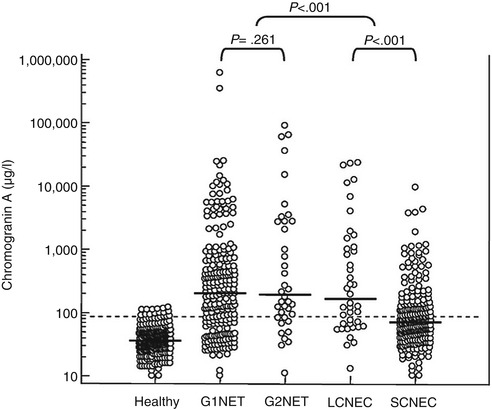

Fig. 7.2
Chromogranin A levels in patients with malignant carcinoid tumours in relation to tumour mass [73]

Fig. 7.3
Chromogranin A levels in patients with tumours with different histological grading. Note that G1 and G2 tumours present higher levels than G3 NEC tumours (LCNEC, SCNEC)
Elevated CgA can also occur in normal individuals and in patients with non-NETs although the levels are usually lower than in patients with NETs. Levels of CgA secretion vary on day-to-day bases and healthy subjects as well as in individuals with NETs. The mean day today variation of CgA is approximately 25 %. Food intake may increase CgA levels; therefore, CgA should be measured in fasting patients to ensure standardisation of the results [30, 31]. It is reported that heart disease and long-term exercise as well as extreme physical stress can elevate CgA. Increased serum or plasma levels of CgA have also been demonstrated in patients with other malignancies including colon, lung, breast, liver and prostate cancer [32]. Other conditions with elevation of CgA are impaired renal function [33], Parkinson disease, untreated hypertension and pregnancy [34], steroid treatment or glucocorticoid excess [35] and chronic atrophic gastritis (type A) and finally treatment with proton-pump inhibitors [36]. Other conditions are inflammatory bowel disease, liver disease and hyperparathyroidism [37]. In renal failure CgA increases due to a decreased plasma clearance, and in autoimmune chronic atrophic gastritis, elevated circulating CgA levels are caused by chronic hypergastrinaemia and stimulation of the ECL cell which proliferate and secrete CgA. A major cause of elevated CgA levels in non-NET patients are the widespread use of PPIs and other suppressive medications. A normalisation of CgA levels occurs by withdrawal of PPI in 1–2 weeks [38].
There is no universal standard calibration for serum or plasma CgA assays. The most useful immunoassays for tumour detection have been those that measure the whole CgA molecule. Assays measuring specifically defined parts of the molecule (pancreastatin) usually have lower sensitivity in detecting patients with NETs. Several commercial available immunoassays (RIAs) and enzyme-linked immunosorbent assays (ELISAs) have been developed for the measurement of circulating CgA concentrations. Moreover, many diagnostic laboratories use in-house assays. It should be noted however that there is a fair amount of variability between assays with sensitivities varying from 67 to 93 % depending on which assay is used. Concerning plasma and serum measurement, a strong positive linear relationship has been reported between plasma and serum CgA values indicating that CgA measurement can be undertaken in both sample types. Measurements of plasma or serum CgA are a valuable tool for both diagnostic and follow-up of patients with NETs [19, 39, 40]. However, there is an unmet need for standardisation of various assays that should be undertaken in a near future.
7.1.1.2 Other Members of the Granin Family (Fig. 7.4)
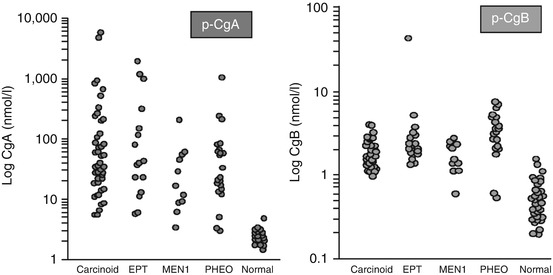
Fig. 7.4
Comparison between circulating levels of chromogranin A and chromogranin B in patients with various NETs. EPT endocrine pancreatic tumours, MEN1 multiple endocrine neoplasia type 1, PHEO pheochromocytomas [73]
Chromogranin B (CgB) is the second most abundant member of the chromogranin family. Like CgA it is a strong acid protein containing approximately 25 % acidic amino acid residues. It has 14 dibasic cleavage points but has been less well studied than CgA. Unlike CgA, CgB does not seem to have increased concentration in patients with renal failure, in patients with atrophic gastritis or those receiving acid-suppressing therapy [21]. In NETs where CgA is not found, CgB may be increased. Such patients include those with MEN-1 and those with tumours in the duodenum or rectum. In addition CgB is a major granin of the human adrenal medulla and may be a more sensitive marker pheochromocytoma [41, 42]. At the moment there is one commercial assay which measures CgB specifically (Euro Diagnostica).
7.1.2 Pancreastatin
CgA is processed by an endoprotease, a pro-hormone convertase 1 (PC1), into smaller peptides such as pancreastatin (PST), a 49-amino acid peptide, that inhibits insulin secretion, somatostatin release, exocrine pancreatic secretion and gastric acid secretion [43–45]. PST is found in human stomach and colon extracts and in liver metastases of gastrinoma. PST has been found to be significantly increased in patients with NETs metastasised to the liver, and the concentrations are proportional to the number of hepatic metastases [41]. PST is not increased in patients with gastric achlorhydria or hypochlorhydria. It may be a very early biomarker to liver tumour activity even when CgA is normal [42].
Recently it has been suggested that analyses of CgA gene expression can be examined by QRT PCR. It has been done in tumour tissue with higher sensitivity than just analysing protein expression that can also be applied in blood. However, data are still lacking [46].
7.1.3 Pancreatic Polypeptide
Pancreatic polypeptide (PP), a 36-amino acid linear peptide, is another general tumour marker secreted by PP cells, which are located in the gut mucosa and pancreas. The uncinate process of the pancreas is particularly rich in PP cells [1]. Other members of the same family are peptide tyrosine-tyrosine (PYY) and neuropeptide Y (NPY). The release of PP is caused by ingestion of meals, particularly those containing protein [41]. It has been found to be elevated in NETs of the gastrointestinal tract and pancreas, with a sensitivity of about 50–80 % [7]. However, there are a lot of clinical conditions where falsely elevated levels are noted, such as diarrhoea, laxative abuse, high age, inflammatory processes in the gut and chronic renal disease [7, 20]. A combination of CgA and PP has been useful in patients with non-functional pNETs, with a sensitivity of almost 95 %. A specific meal stimulatory test (mixed meal) has been particularly useful in patients with multiple endocrine neoplasia type 1 (MEN-1) and early detects pancreatic tumours [21].
7.1.4 Neuron-Specific Enolase (NSE)
NSE is the neuron-specific isomer of the glycolytic enzyme 2-phospho-d-glycerate hydroxylase or enolase. This isomer is present in the cytoplasm of neurons and neuroendocrine cells and can serve as a circulating marker for NETs. NSE is most frequent elevating in patients with small-cell lung cancer but has also been found to be elevated in 30–50 % patients with intestinal NETs, medullary thyroid carcinoma, pNETs and pheochromocytomas [25, 42]. In patients with poorly differentiated NETs, NSE might be elevated despite normal CgA. NSE is also roughly correlated with tumour size although specificity is lower than that of CgA [25]. The combination of CgA and NSE has a higher sensitivity than either parameter separately [7, 14, 21]. Recently an early response in NSE is related to therapeutic response with an mTOR inhibitor (everolimus) [29].
7.1.5 Human Chorionic Gonadotropin (HCG)
Human chorionic gonadotropin (HCG), a glycoprotein hormone consisting of alpha and beta subunits, can be ectopically produced by neoplasms. Assays for the intact glycoprotein and its alpha and beta subunits have been used as markers to screen for a number of different tumours including NETs. In particular, HCG alpha and beta subunits have been found to be increased in patients with malignant pNETs [47, 48].
7.1.6 Other General Tumour Markers
Pro-gastrin-releasing peptide (Pro-GRP) is a promising tumour marker for small-cell lung cancer. Pro-GRP is the precursor of the neuropeptide gastrin-releasing peptide (GRP). Its production is increased in small-cell lung cancer [49]. It has been described that levels are elevated in patients with NETs, particularly those with high-grade tumours (G3) [25]. Cytokeratin fragments (CKfr) are sensitive indicators of tumour cell turnover and are especially useful in the management of patients with malignancies of epithelial origin [50]. CKfr is associated with angiogenesis factors which may play a role in NETs [51]. However, limited data are so far presented. The assay currently used is determining CKfr 8, 18 and 19 fragments in serum [25]. In an attempt to look at the choice of general tumour markers in patients with NETs in relation to histological grade, Korse and co-workers have analysed CgA neuron-specific enolase (NSE) pro-GRP and CKfr [25]. The largest area under the ROC curve was for CgA in patients with G1 and G2 NETs. Pro-GRP showed the highest sensitivity (73 %) and 95 % specificity in patients with small-cell NETs (G3). In a multivariate survival analysis, only CKfr was associated with survival (p < 0.0001) for patients with well-differentiated NETs (G1 and G2 NETs). In patients with poorly differentiated NEC (G3), both CKfr and NSE were associated with survival (p < 0.001 and p = 0.003, respectively). The authors recommend in patients with well-differentiated NETs (G1, G2) the use of CgA and CKfr, whilst in patients with NEC G3, pro-GRP and CKfr and NSE are preferred (Fig. 7.5).
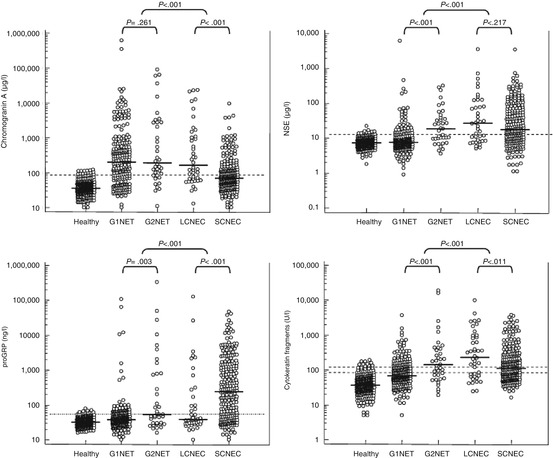

Fig. 7.5
Comparison of different biochemical markers in a material of NETs with different histological grade [25]
7.2 Specific Markers and Related Tumours (Table 7.3)
Table 7.3
Site-specific markers
Site | Biomarkers | Specificity |
|---|---|---|
Thymus | ACTH | I |
Lung | ACTH, ADH, serotonin, 5-HIAA | I |
Histamine, GRP, GHRH, VIP, PTHrp | L | |
Stomach | Histamine, gastrin | I |
Ghrelin | L | |
Pancreas | Gastrin, insulin, proinsulin, glucagon, somatostatin | H |
C-peptide, neurotensin, VIP, PTHrp, calcitonin | L | |
Duodenum | Somatostatin, gastrin | H |
Ileum | Serotonin, 5-HIAA | H |
NKA, neuropeptide K, SP | I | |
Colorectum | Peptide YY, somatostatin | I |
In addition to general markers, there are biomarkers specific for the different functioning NETs. The various tumours and secretarial products are summarised in Table 7.3.
Carcinoid tumours have traditionally been divided according to the presumed embryological origin of the precursor cell thus divided into the foregut (lung, thymus, stomach and duodenum), midgut (jejunum, ileum, appendix and caecum) and hindgut (distal colon and rectum) carcinoids and together with pNETs collectively considered as gastroenteropancreatic NETs [4, 7, 14, 20]. This old classification has now been abandoned, and a new classification established by WHO is now in clinical practice. Well-differentiated endocrine tumours, WHO G1 tumours, are characterised by a low grade of proliferation, with Ki-67 < 2 %. Well-differentiated endocrine tumours, WHO G2, Ki-67 between 3 and 20 % and poorly differentiated endocrine carcinoma with high grade of proliferation Ki-67 > 20 % (NEC G3) [2, 51–53]. Besides this classification NETs are also divided into functional tumours, which produce a clinical hormone-related syndrome, versus non-functional tumours which might present symptoms related to tumour growth [4, 20].
7.2.1 Bronchial and Thymic NETs
Bronchial NETs are essentially divided into typical, atypical, large-cell and small-cell lung NETs. This paragraph will concentrate on typical and atypical lung NETs. These tumours can produce almost any of the described hormones or amines in the body. The carcinoid syndrome including flushing, diarrhoea, cardiac fibrosis, wheezing and dyspnoea occurs in lung NETs but is usually a atypical variant and not quite similar to the syndrome in the small intestine. The syndrome is rarely related to production of serotonin and tachykinins but because of the lack of enzymes converting hydroxytryptophan (5-HTP) to serotonin patients show a prolonged flushing, headache, palpitation and lacrimation as well as broncho-constriction [4, 7]. Lung NETs may also produce Cushing syndrome due to ectopic adrenocorticotrophic hormone (ACTH) production or acromegaly due to ectopic growth hormone-releasing hormone (GHRH) secretion [4, 7, 14]. Besides these specific biomarkers, these tumours also produce CgA as well as prepro-gastrin and NSE. Thymic carcinoids are usually of the non-functioning type, but about 30 % of the patients can present the Cushing syndrome with increased secretion of ACTH or corticotrophin-releasing factor (CRF) [54–56].
7.2.2 Gastro-duodenal NETs
Duodenal NETs (carcinoids) may secret gastrin and cause the typical Zollinger-Ellison syndrome with recurrent ulcers, diarrhoea and abdominal pain [20, 21]. The duodenal gastrinoma may be part of MEN-1 syndrome. Somatostatin-producing NETs are also found in the duodenum. These are usually not presenting any hormone-related symptoms, but histologically they present with psammoma bodies rather than the presence of somatostatinoma syndrome. Some of these tumours are part of the von Hippel-Lindau disease (VHL) [1–3]. Gastric NETs are divided into ECLoma type 1, 2 and 3 and are derived from ECL cells in the corpus fundus region of the stomach. Another type of gastric NETs is G-cell tumours of the antrum. Most antral carcinoids are producing not only gastrin but also ghrelin and sometimes serotonin [57, 58]. The type 1 ECL tumours are associated with chronic atrophic gastritis and present high levels of CgA and sometimes also histamine production and the atypical carcinoid syndrome. Type 2 tumours are associated with MEN-1, Zollinger-Ellison syndrome and hypertrophic gastropathy. Type 3 tumours are sporadic and are not associated with any distinctive gastric pathology. Type 1 and 2 tumours are sharing common hypergastrinaemia, whereas type 3 tumours are independent of any overt hormonal imbalance. All three subtypes of ECL tumours present elevated plasma chromogranin A levels [59–61].
7.2.3 Small Intestinal Neuroendocrine Tumours (Midgut Carcinoids)
Carcinoid syndrome is a typical clinical presentation of metastatic ileal carcinoid occurring in about 18 % of patients and is characterised by flushing, diarrhoea and abdominal pain [3, 4]. Less frequent events are carcinoid heart disease as well as broncho-constriction and pellagra-like skin changes. The syndrome is related to massive release of serotonin which is no longer metabolised in the liver. Other substances involved in the carcinoid syndrome might be tachykinins (neuropeptide K, substance B, NKA), prostaglandins and bradykinins [6, 7, 14]. Patients with carcinoid syndrome present with increased levels in the urine of the breakdown product of serotonin 5-HIAA, with levels between 100 and 3,000 μmol/24 h (ref. <50 μmol/24 h). The overall sensitivity and specificity of u-5-HIAA in the presence of carcinoid syndrome is 70 and 90 %, respectively [4, 7, 14, 62]. Therefore, this marker is the most frequently performed assay in the clinical setting of the carcinoid syndrome. High-pressure liquid chromatography (HPLC) with electrochemical detection is currently recommended to measure urinary 5-HIAA. In some laboratories automated assays using mass spectrometry are available. It has been recently suggested that plasma 5-HIAA might be a valid alternative to the collection of u-5-HIAA [63]. Some studies have demonstrated higher sensitivity (100 %) for the measurement of platelet poor plasma serotonin compared with u-5-HIAA in patients with small intestinal NETs [14]. However, there are large fluctuations in the plasma levels of serotonin depending upon food ingestion, time of the day, stress and sampling procedures. In addition, certain malabsorptive conditions including Whipple’s disease and celiac sprue may be associated with elevated urine 5-HIAA levels. It is also important that the patient gets strict diet instructions, avoiding plums, pineapples, bananas, eggplants, tomatoes, avocados and walnuts during the collection of urine. Certain medications made also interfere with the assay: paracetamol, fluorouracil, methysergide and naproxen. On the contrary levodopa, aspirin, ACTH, methyldopa and phenothiazine may give false-negative results. Somatostatin analogues are known to decrease levels of 5-HIAA. Serotonin plays a key role in the development of peritoneal and cardiac fibrosis with activation of the 5-HT2B receptor and the cascade of connected tissue growth factors. Reduction in plasma serotonin levels correlated with decreased incidence of carcinoid heart disease. Moreover, u-5-HIAA excretion also correlates with the severity of carcinoid heart disease and prognosis in patients with carcinoid syndrome. In the clinical practice, it is useful to have two consecutive 24 h collection of urine for measurements of 5-HIAA and take the mean value, but there is a strong argument to change to measurements of plasma 5-HIAA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree