3
Coma
Coma is a sleep-like state in which the patient makes no purposeful response to the environment and from which he or she cannot be aroused. The eyes are closed and do not open spontaneously. The patient does not speak, and there is no purposeful movement of the face or limbs. Verbal stimulation produces no response. Painful stimulation may produce no response or nonpurposeful reflex movements mediated through spinal cord or brainstem pathways.
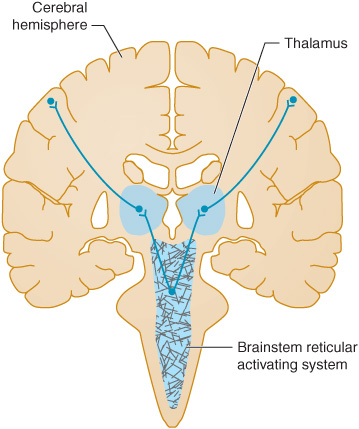
Coma results from a disturbance in the function of either the brainstem reticular activating system above the mid pons or of both cerebral hemispheres (Figure 3-1), as these are the brain regions that maintain consciousness.
Figure 3-1. Anatomic basis of coma. Consciousness is maintained by the normal functioning of the brainstem reticular activating system above the mid pons and its bilateral projections to the thalamus and cerebral hemispheres. Coma results from lesions that affect either the reticular activating system or both hemispheres.
APPROACH TO DIAGNOSIS
The approach to diagnosis of the comatose patient consists first of emergency measures to stabilize the patient and treat presumptively certain life-threatening disorders, followed by efforts to establish an etiologic diagnosis.
EMERGENCY MANAGEMENT
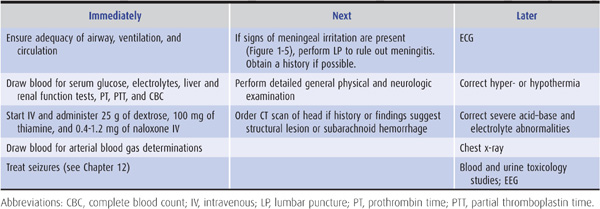
As summarized in Table 3-1, emergency management of the comatose patient includes the following steps:
1. Ensure patency of the airway and adequacy of ventilation and circulation. This is accomplished by rapid visual inspection and by measuring the vital signs. If the airway is obstructed, the obstruction should be cleared and the patient intubated. If there is evidence of trauma that may have affected the cervical spine, however, the neck should not be moved until stability has been established by x-rays of the cervical spine. If spinal instability is present and intubation is required, tracheostomy should be performed. Adequacy of ventilation can be established by the absence of cyanosis, a respiratory rate greater than 8/min, the presence of breath sounds on auscultation of the chest, and the results of arterial blood gas and pH studies (see later). If any of these suggest inadequate ventilation, the patient should be ventilated mechanically. Measurement of the pulse and blood pressure provides a rapid assessment of the status of the circulation. Circulatory embarrassment should be treated with intravenous fluid replacement, pressors, and antiarrhythmic drugs, as indicated.
Table 3-1. Emergency management of the comatose patient.
2. Insert an intravenous catheter and withdraw blood for laboratory studies. These studies should include measurement of serum glucose and electrolytes, hepatic and renal function tests, prothrombin time, partial thromboplastin time, and a complete blood count. Extra tubes of blood should also be obtained for additional studies that may be useful in certain cases, such as drug screens, and for tests that become necessary as diagnostic evaluation proceeds.
3. Begin an intravenous infusion and administer dextrose, thiamine, and naloxone. Every comatose patient should be given 25 g of dextrose intravenously, typically as 50 mL of a 50% dextrose solution, to treat possible hypoglycemic coma. Because administration of dextrose alone may precipitate or worsen Wernicke encephalopathy (see Chapter 4) in thiamine-deficient patients, all comatose patients should also receive 100 mg of thiamine by the intravenous route. To treat possible opiate overdose, the opiate antagonist naloxone, 0.4 to 1.2 mg intravenously, should also be administered routinely to comatose patients. The benzodiazepine antagonist flumazenil, 1 to 10 mg intravenously, may be useful when benzodiazepine overdose contributes to coma. However, it should not be used in patients with a history of seizures, chronic benzodiazepine abuse, or suspected coingestion of tri- or tetracyclic antidepressants. The latter should be suspected if the electrocardiogram (ECG) shows sinus tachycardia at a rate of >130/min, QTc interval >0.5 seconds, and QRS duration >0.1 seconds.
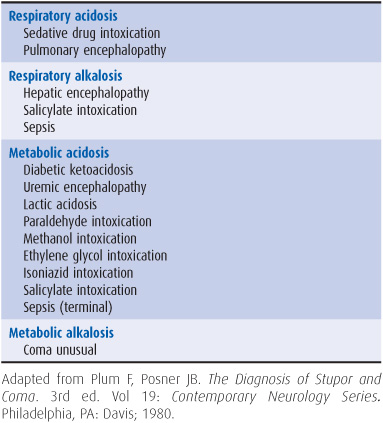
4. Withdraw arterial blood for blood gas and pH determinations. In addition to assisting in the assessment of ventilatory status, these studies can provide clues to metabolic causes of coma (Table 3-2).
Table 3-2. Metabolic coma: differential diagnosis by acid–base abnormalities.
5. Institute treatment for seizures, if present. Persistent or recurrent seizures in a comatose patient should be considered to represent status epilepticus and treated accordingly, as described in Chapter 12 (see particularly Table 12-6).
After these measures have been taken, the history (if available) is obtained, and general physical and neurologic examinations are performed.
HISTORY & EXAMINATION
HISTORY
The most crucial aspect of the history is the time over which coma develops. In the absence of precise details about the mode of onset, information about when the patient was last seen in an apparently normal state may assist in establishing the time course of the disease process.
1. A sudden onset of coma suggests a vascular origin, especially a brainstem stroke or subarachnoid hemorrhage.
2. Rapid progression from hemispheric signs, such as hemiparesis, hemisensory deficit, or aphasia, to coma within minutes to hours is characteristic of intracerebral hemorrhage.
3. A more protracted course leading to coma (days to a week or more) is seen with tumor, abscess, or chronic subdural hematoma.
4. Coma preceded by a confusional state or agitated delirium, without lateralizing signs or symptoms, is probably due to a metabolic derangement or infection (meningitis or encephalitis).
GENERAL PHYSICAL EXAMINATION
Signs of Trauma
1. Inspection of the head may reveal signs of basilar skull fracture, including the following:
A. Raccoon eyes—Periorbital ecchymoses (see Figure 1-4).
B. Battle sign—Swelling and discoloration overlying the mastoid bone behind the ear (see Figure 1-4).
C. Hemotympanum—Blood behind the tympanic membrane.
D. Cerebrospinal fluid (CSF) rhinorrhea or otorrhea—Leakage of CSF from the nose or ear. CSF rhinorrhea must be distinguished from other causes of rhinorrhea, such as allergic rhinitis. Glucose concentration does not reliably distinguish CSF from nasal mucus, but beta-2 transferrin is unique to CSF, and its presence documents a CSF source of rhinorrhea.
2. Palpation of the head may demonstrate a depressed skull fracture or swelling of soft tissues at the site of trauma.
Blood Pressure
Elevated blood pressure in a comatose patient may reflect long-standing hypertension, which predisposes to intracerebral hemorrhage or stroke. In the rare condition of hypertensive encephalopathy, the blood pressure is above 250/150 mm Hg in chronically hypertensive patients; it may be lower in children or after acute elevation of blood pressure in previously normotensive patients (eg, in acute renal failure). Elevated blood pressure may also be a consequence of the process causing the coma, as in intracerebral or subarachnoid hemorrhage or, rarely, brainstem stroke.
Temperature
Hypothermia occurs in coma caused by ethanol or sedative drug intoxication, hypoglycemia, Wernicke encephalopathy, hepatic encephalopathy, and myxedema. Coma with hyperthermia is seen in heat stroke, status epilepticus, malignant hyperthermia related to inhalational anesthetics, anticholinergic drug intoxication, pontine hemorrhage, and certain hypothalamic lesions.
Signs of Meningeal Irritation
Signs of meningeal irritation (eg, nuchal rigidity or the Brudzinski sign [see Figure 1-5]) can be invaluable in the prompt diagnosis of meningitis or subarachnoid hemorrhage, but these signs are lost in deep coma, so their absence does not exclude these conditions.
Optic Fundi
Examination of the optic fundi may reveal papilledema or retinal hemorrhages compatible with chronic or acute hypertension, or an elevation in intracranial pressure (Figure 1-11). Subhyaloid (superficial retinal) hemorrhages in an adult strongly suggest subarachnoid hemorrhage (Figure 6-3).
NEUROLOGIC EXAMINATION
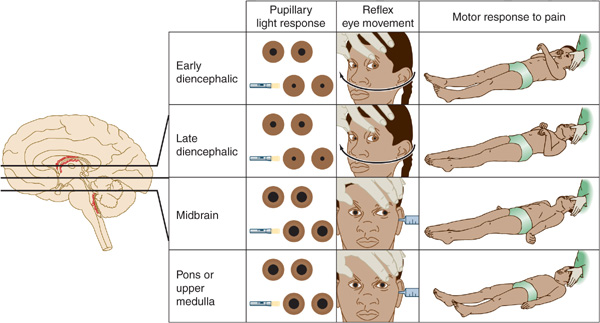
The neurologic examination is the key to etiologic diagnosis in the comatose patient. Pupillary size and reactivity, reflex eye movements (oculocephalic and oculovestibular reflexes), and the motor response to pain should be evaluated in detail (Figure 3-2).
Figure 3-2. Neurologic signs in coma with downward transtentorial herniation. In the early diencephalic phase, the pupils are small (approximately 2 mm in diameter) and reactive, reflex eye movements are intact, and the motor response to pain is purposeful or semipurposeful (localizing) and often asymmetric. The late diencephalic phase is associated with similar findings, except that painful stimulation results in decorticate (flexor) posturing, which may also be asymmetric. With midbrain involvement, the pupils are fixed and midsized (approximately 5 mm in diameter), reflex adduction of the eyes is impaired, and pain elicits decerebrate (extensor) posturing. Progression to involve the pons or medulla also produces fixed, midsized pupils, but these are accompanied by loss of reflex abduction as well as adduction of the eyes and by no motor response or only leg flexion upon painful stimulation. Note that although a lesion restricted to the pons produces pinpoint pupils as a result of the destruction of descending sympathetic (pupillodilator) pathways, downward herniation to the pontine level is associated with midsized pupils. This happens because herniation also interrupts parasympathetic (pupilloconstrictor) fibers in the oculomotor (III) nerve.
Pupils
1. Normal pupils—Normal pupils are typically 3 to 4 mm in diameter (but larger in children and smaller in the elderly) and equal in size bilaterally; they constrict briskly and symmetrically in response to light. Normally reactive pupils in a comatose patient are characteristic of a metabolic cause.
2. Thalamic pupils—Slightly smaller (~2 mm) reactive pupils are present in the early stages of thalamic compression from mass lesions, perhaps because of interruption of the descending sympathetic pathways.
4. Fixed, midsized pupils—Pupils fixed at approximately 5 mm in diameter are the result of brainstem damage at the midbrain level, which interrupts both sympathetic, pupillodilator and parasympathetic, pupilloconstrictor nerve fibers.
5. Pinpoint pupils—Pinpoint pupils (1-1.5 mm in diameter) in a comatose patient usually indicate opioid overdose or, less commonly, a focal structural lesion in the pons. These causes can be distinguished by the administration of naloxone (see earlier) or by the associated defects in horizontal eye movements that usually accompany pontine lesions. Pinpoint pupils may appear unreactive to light except when viewed with a magnifying glass. Pinpoint pupils can also be caused by organo-phosphate poisoning, miotic eye drops, or neurosyphilis (Argyll Robertson pupils).
6. Asymmetric pupils—Asymmetry of pupillary size (anisocoria) with a difference of 1 mm or less in diameter is a normal finding that occurs in 20% of the population. In such physiologic anisocoria, pupils constrict to a similar extent in response to light, and extraocular movements are unimpaired. In contrast, a pupil that constricts less rapidly or to a lesser extent than its contralateral fellow usually implies a structural lesion affecting the midbrain, oculomotor nerve, or eye.
Eye Movements
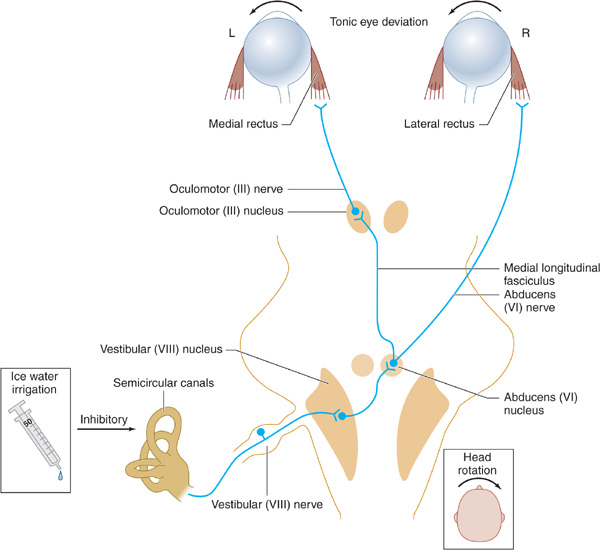
1. Pathways tested—The neuronal pathways examined by testing eye movements begin at the pontomedullary junction (vestibular [VIII] nerve and nucleus), synapse in the caudal pons (horizontal gaze center and abducens [VI] nerve nucleus), ascend through the central core of the brainstem reticular activating system (medial longitudinal fasciculus), and arrive at the contralateral mid-brain (oculomotor [III] nucleus and nerve; Figure 3-3).
Figure 3-3. Brainstem pathways mediating reflex conjugate horizontal eye movements. In a comatose patient with intact brainstem function, irrigation of the tympanic membrane with ice water inhibits the vestibuloocular pathways shown, resulting in tonic deviation of both eyes toward the irrigated side; head rotation causes eye deviation away from the direction of rotation.
2. Methods of testing—In the comatose patient, eye movements are tested by stimulating the vestibular system (semicircular canals of the middle ear) by passive head rotation (the oculocephalic reflex, or doll’s-head maneuver) or using the stronger stimulus of ice-water irrigation against the tympanic membrane (oculovestibular reflex, or cold-water caloric testing) (Figure 3-3).
The doll’s-head (oculocephalic) maneuver is performed by rotating the head horizontally to elicit horizontal eye movements and vertically to elicit vertical movements. The eyes should move in the direction opposite to that of head rotation. This may be an inadequate stimulus for inducing eye movements, however, and the reflex may be overridden in conscious patients.
Cold-water caloric (oculovestibular) stimulation is a more potent stimulus and is performed by irrigating the tympanic membrane with ice water. Otoscopic examination should always be undertaken before this maneuver is attempted, because it is contraindicated if the tympanic membrane is perforated. In conscious patients, unilateral cold water irrigation produces nystagmus with the fast phase directed away from the irrigated side. In comatose patients with intact brainstem function, unilateral ice water irrigation results in tonic deviation of the eyes toward the irrigated side. Bilateral irrigation with ice water causes tonic downward deviation, whereas bilateral stimulation with warm (44°C) water induces tonic upward deviation. An absent or impaired response to caloric stimulation with large volumes (eg, 50 mL) of ice water is indicative of peripheral vestibular disease, a structural lesion involving the posterior fossa (cerebellum or brainstem), or intoxication with sedative drugs.
3. Normal movements—A comatose patient with intact brainstem function has full conjugate horizontal eye movements, which occur either spontaneously (as “roving eye movements”) or during the doll’s-head maneuver, as well as tonic conjugate deviation of both eyes toward the side of the ice-water irrigation during cold-water caloric testing. Full horizontal eye movements in a comatose patient exclude a structural lesion in the brainstem as the cause of coma and suggest either a nonstructural (eg, metabolic) cause or, less commonly, bilateral hemispheric lesions.
4. Abnormal movements
a. With lesions affecting the oculomotor (III) nerve or nucleus, such as hemispheric mass lesions causing downward transtentorial herniation (Figure 3-2), cold-water caloric testing fails to produce adduction of the ipsilateral eye, whereas the contralateral eye abducts normally.
b. Complete unresponsiveness to cold-water caloric testing in a comatose patient implies either a structural lesion of the brainstem affecting the pons or a metabolic disorder that affects the brainstem preferentially, such as sedative drug intoxication.
c. Downward deviation of one or both eyes in response to unilateral cold-water caloric testing also suggests sedative drug intoxication.
Motor Response to Pain
The motor response to pain is assessed by applying strong pressure on the supraorbital ridge, sternum, or nail beds. The response to such stimuli can indicate whether the condition causing coma affects the brain symmetrically (as is typical of metabolic and other diffuse disorders) or asymmetrically (as in unilateral structural lesions). The motor response to pain may also help to localize the anatomic level of cerebral dysfunction or provide a guide to the depth of coma.
1. With cerebral dysfunction of only moderate severity, patients may localize an offending stimulus by reaching toward the site of stimulation. Although such “semipurposeful” localizing responses can be difficult to distinguish from the reflex responses described later, movements that involve limb abduction almost never represent reflexes.
2. A decorticate response to pain (flexion of the arm at the elbow, adduction at the shoulder, and extension of the leg and ankle) is classically associated with lesions that involve the thalamus directly or large hemispheric masses that compress the thalamus from above.
3. A decerebrate response (extension at the elbow, internal rotation at the shoulder and forearm, and leg extension) tends to occur when brain dysfunction has descended to the level of the midbrain. Thus decerebrate posturing generally implies more severe brain dysfunction than decorticate posturing, although neither response localizes the site of dysfunction precisely.
4. Bilateral symmetric posturing may be seen in both structural and metabolic disorders.
5. Unilateral or asymmetric posturing suggests structural disease in the contralateral cerebral hemisphere or brainstem.
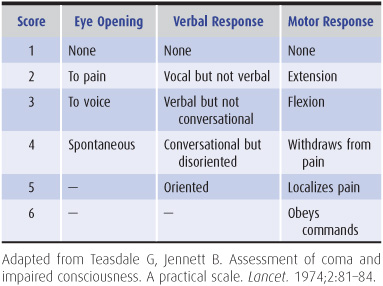
Glasgow Coma Scale
The pupillary, eye movement, and motor responses described earlier are sometimes translated to a numerical scale so that changes in the examination (and thus the numerical score) may be more easily noticed over time and compared between different examiners (Table 3-3).
Table 3-3. Glasgow coma scale.
PATHOPHYSIOLOGIC ASSESSMENT
The most important step in evaluating a comatose patient is to decide whether the cause is a structural brain lesion (for which emergency neurosurgical intervention may be required) or a diffuse disorder caused by a metabolic disturbance, meningitis, or seizures (for which immediate medical treatment may be needed).
Supratentorial Structural Lesions
When coma is the result of a supratentorial mass lesion, the history and physical findings early in the course usually point to dysfunction of one cerebral hemisphere. Symptoms and signs include contralateral hemiparesis, contralateral hemisensory loss, aphasia (with dominant, usually left, hemisphere lesions), and agnosia (indifference to or denial of the deficit, with injury to the nondominant hemisphere).
As the mass expands (commonly from associated edema), the patient becomes increasingly lethargic due to compression of the contralateral hemisphere or thalamus. Stupor progresses to coma, but findings on examination often remain asymmetric. With rostral–caudal (downward) progression of brain injury, the thalamus, midbrain, pons, and medulla become sequentially involved, and the neurologic examination reveals dysfunction at successively lower anatomic levels (Figure 3-2). This segmental pattern of involvement strongly supports the diagnosis of a supratentorial mass with downward transtentorial herniation (Figure 3-4) and dictates the need for neurosurgical intervention. At the fully developed midbrain level (midsized, unreactive pupils), chances of survival without severe neurologic impairment decrease rapidly, especially in adults. Once the pontine level of dysfunction is reached (unreactive pupils, and absent horizontal eye movements), a fatal outcome is inevitable.
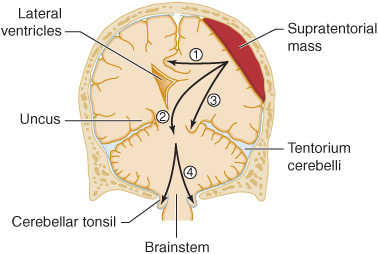
Figure 3-4. Anatomic basis of herniation syndromes. An expanding supratentorial mass lesion may cause brain tissue to be displaced into an adjacent intracranial compartment, resulting in (1) cingulate herniation under the falx, (2) downward transtentorial (central) herniation, (3) uncal herniation over the edge of the tentorium, or (4) cerebellar tonsillar herniation into the foramen magnum. Coma and ultimately death result when (2), (3), or (4) produces brainstem compression.
Supratentorial mass lesions may cause herniation of the medial portion of the temporal lobe (the uncus) over the edge of the cerebellar tentorium (Figure 3-4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree