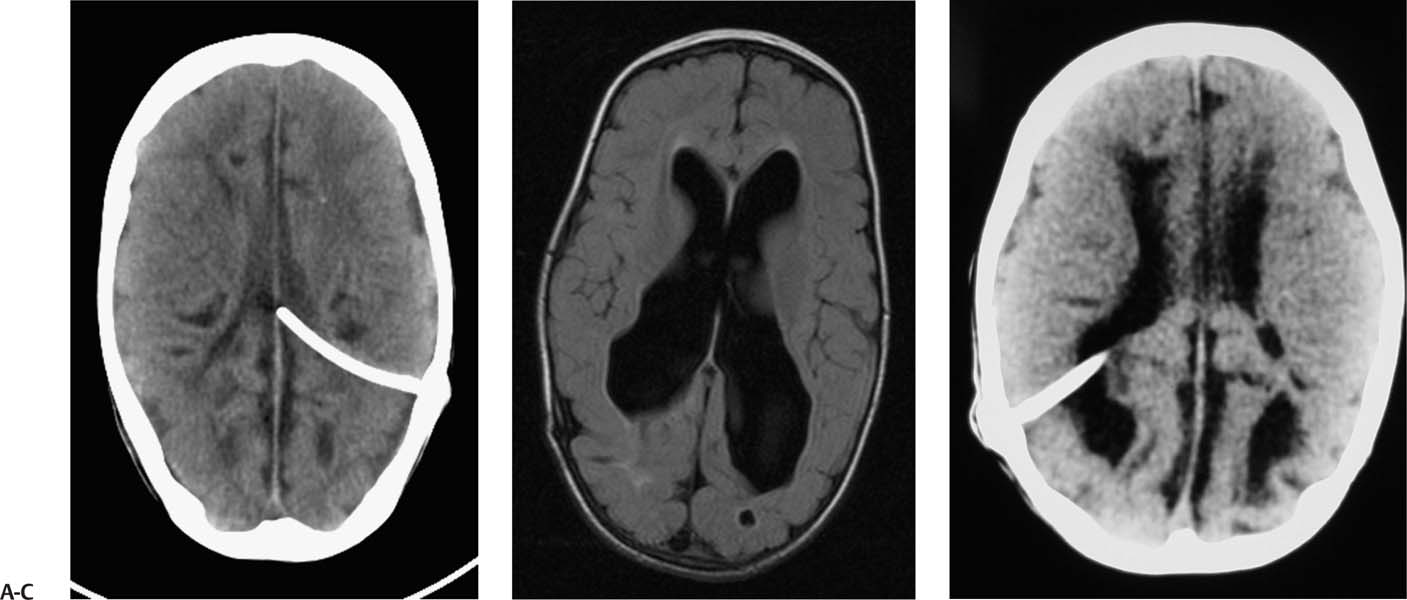
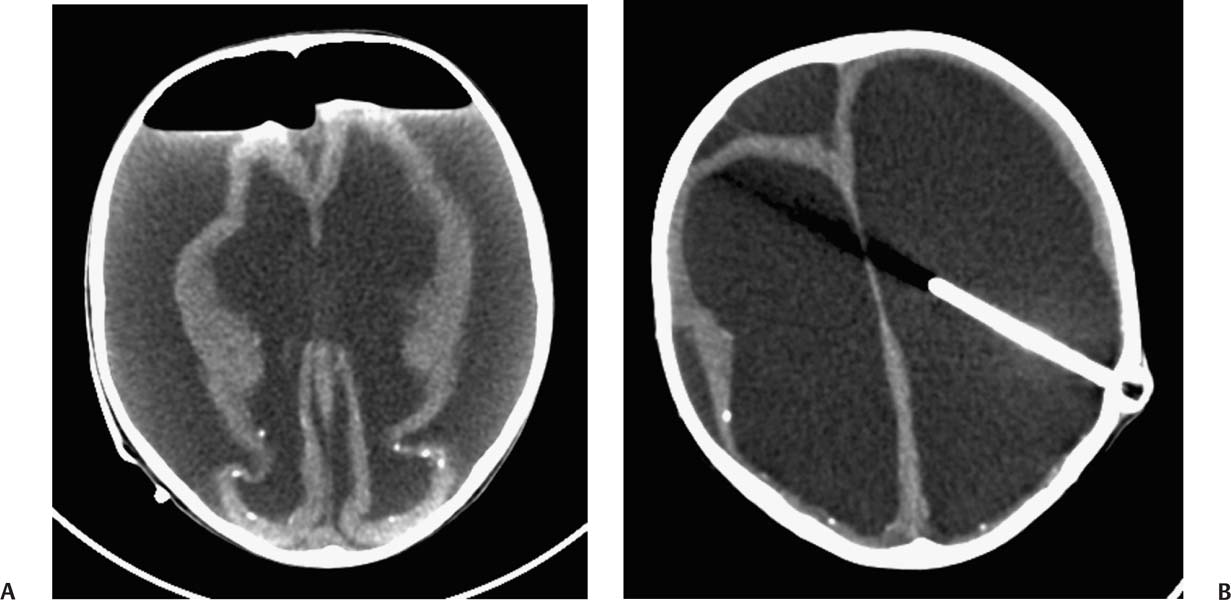
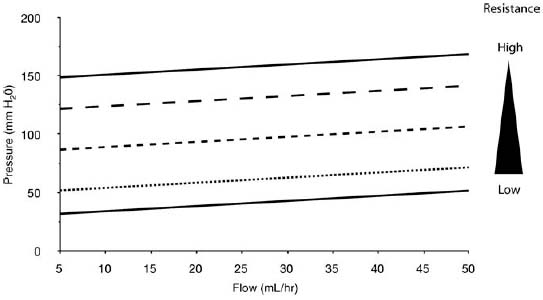
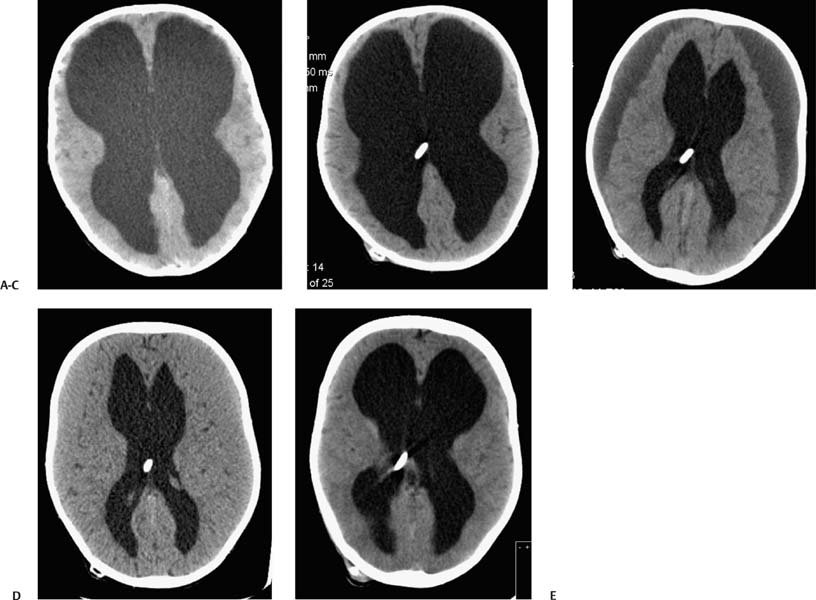
2 Communicating Hydrocephalus The introduction of ventricular shunts in the early 1950s1revolutionized the treatment of hydrocephalus and converted it from a terminal disease2 to a treatable condition with overall good physical and cognitive outcome.3–8 These early shunts contained a one-way valve, which opened and allowed passage of cerebrospinal fluid (CSF) when the difference of pressure between its input and output reached a preset threshold level. This type of valve is now referred to as pressure-regulated or differential pressure and in its most common implementation, which has been marketed for 4 decades, contains a silicone diaphragm mechanism that bends under pressure and opens circumferentially a narrow passage through which CSF escapes. Other designs also exist with similar pressure characteristics, for example, proximal or distal slit, “spring-ball,” “ball-in-cone,” “duck-bill,” cruciform, and diamond slit.9 In all designs, the deformation characteristics of an elastic material are taken advantage of to create a one-way mechanism that opens whenever the proximal (ventricular) pressure exceeds a preset level. This preset pressure level is intended to simulate normal intracranial pressure (ICP) within the cerebral ventricular system; hence it is set around 10 to12 cm H20, the nominal normal ICP. Early on it was appreciated that different patients may respond better clinically if this preset pressure was higher or lower than the average “medium” setting; thus, “high” and “low” pressure valves were designed. Despite tremendous technological improvements over the last several decades, there has been little change in shunt valve design in essence, if one excludes the improvement in materials, that has had a measurable effect on the long-term mechanical complications of shunts. A major breakthrough came in the 1980s, when the syndrome of shunt overdrainage due to “siphoning” (rapid loss of CSF from the head) in the erect position was appreciated. Different technological solutions were pursued to overcome this. Two main types of devices were developed: “antisiphon” or “siphon control” devices and “flow control” valves. Antisiphon devices come in different designs; common ones use either a dome to sense atmospheric pressure and apply increased resistance to flow in the erect position (e.g., Delta chamber, Medtronic PS Medical, Goleta, California) or gravity-controlled balls that progressively occlude the CSF passage and increase the resistance to flow (e.g., ShuntAssistant, Christoff Miethke GmbH & Co. KG, Potsdam, Germany; and SiphonGuard, Codman & Shurtleff Inc., Raynham, Massachusetts). Most manufacturers have incorporated differential pressure valves and antisiphon devices in single-case shunts (e.g., Delta valve = differential pressure valve + Delta chamber, Medtronic PS Medical). Flow control valves provide constant flow of CSF over a wide range of pressures. The best example is the Orbis-Sigma OSV II valve (Integra LifeSciences Corp., Plainsboro, New Jersey), which contains a dome connected to a movable ruby pin with variable profile that allows a stable flow of CSF to a wide pressure range, while including an overflow mechanism in case the CSF pressure becomes too high. Recently, the OSV valve was developed in a lower flow setting as well, to suit patients with normal pressure hydrocephalus (NPH) who may be more prone to chronic subdural hematomas following shunting. In the 1980s, an adjustable valve was produced, the HakimMedos programmable valve (now called the Codman Hakim programmable valve, Codman & Shurtleff), which was able to change the opening pressure setting percutaneously with the help of a magnet system. It offered the facility to change the opening pressure in 18 steps of 10 mm H20 over a wide range, from 30 to 200 mm H20. It was an interesting concept, certainly advanced for its time. The Codman Hakim was marketed as a programmable valve, but it is better thought of as adjustable, as the user can change only its opening pressure, not its essential functional characteristics; in other words, it operates on only one program, at different pressure levels. The Codman Hakim is a differential pressure valve, with a ruby ball and seat controlled by a stainless steel spring of adjustable height. Although its market acceptance (by market, we mean neurosurgeons worldwide who implant shunts) was slow, it is still in production today in its original form. Over the past 5 years the concept of changeable opening pressure has gained acceptance. Other adjustable valve designs have appeared as well, and they are gradually gaining market acceptance or market share. Incidentally, the use of financial terms when discussing scientific devices like shunts is appropriate, because the technological evolution that became available for clinical use is directly related to the economic realities that apply to the manufacture and distribution of these devices.10,11 Recently, manufacturers have combined adjustable pressure/differential pressure valves with antisiphon devices in a single case. Three notable examples are the Strata valve (Medtronic PS Medical), an adjustable Delta valve (diaphragm differential pressure valve + Delta chamber) with five pressure settings; the Miethke Pro-GAV (Christoff Miethke GmbH & Co. KG, Potsdam, Germany), which combines a ball-in-cone differential pressure valve and a gravity-controlled ball antisiphon device; and the Codman Hakim programmable valve, which has been combined with the SiphonGuard (Codman & Shurtleff) in the same case. These newer devices offer improved laboratory characteristics but have not been available long enough to ascertain their clinical performance. Flow control valves are not yet available in adjustable models. After 5 decades of shunt design evolution, laboratory and clinical studies, and considerable scientific debate at countless meetings, the issues of superiority of shunt valve design remain unresolved. This by implication signifies the difficulties with the development of implantable medical devices, as well as the economies of scale that affect medical devices in comparison with commercial electronic goods, for example, which incorporate infinitely more complicated technology than shunt valves and are sold for a fraction of the price. At the same time, it has been rather striking how polarized neurosurgeons’ views are on the choice of shunts, though no conclusive scientific evidence exists, which implies that significant factors in the choice of shunts play to the history of education of each individual surgeon (most surgeons choose valves that their mentors were using when they were training, in parallel to the known assertion in commerce that most drivers will choose the same make of car when they change, provided they had no major problems with the previous one), and the ability of the industry to penetrate the local environment of neurosurgeons using the usual market techniques (advertisements, personal contacts, social trends, etc.). Several in vitro studies have examined the hydrodynamic properties of shunt valves, with particular reference to antisiphon action.12,13 Two major valve-testing facilities exist: in Cambridge, UK, and in Heidelberg, Germany, in the respective Departments of Neurosurgery. Both of these testing facilities have consistently shown that most shunt valves do not counteract effectively the overdrainage phenomenon in the erect position, despite manufacturers’ claims.12,13 The standard differential pressure valves have major susceptibilities to overdrainage, the Codman Hakim programmable valve cannot counteract overdrainage in the erect position even in its higher settings, and the dome-designed antisiphon devices (e.g., Delta chamber, Medtronic PS Medical) are susceptible to subcutaneous pressure.14 This last problem has been shown to render the devices inactive with time, as they are implanted in the subcutaneous tissue, which encases them in fibrous tissue, and they lose their reference to atmospheric pressure, which deactivates the whole mechanism of increased resistance to CSF flow. Considerable controversy exists still on whether flow-regulated valves offer a better clinical long-term outcome than pressure regulated devices. Several clinical studies and a randomized clinical trial have not yet proven this conclusively. It seems that, although in the laboratory, flow-regulated valves are better than differential pressure valves in counteracting the siphoning effect and maintaining normal ICP, in actual clinical practice, the overall shunt survival time (the time from implantation to the shunt’s first revision due to some complication, commonly obstruction) is not substantially different between the two shunt types, the only difference being the types of complications that affect them.15–17 Differential pressure valves are associated with a higher proximal obstruction rate, whereas flow control valves are associated with a higher valve obstruction rate. Furthermore, differential pressure valves are associated with a higher incidence of slit ventricles seen on radiology, but only a minority of patients develop the clinical features of the syndrome (Fig. 2.1). Cost comparison studies, performed mainly for the realities of the developing world, have shown no difference in clinical outcome in differential pressure valves costing $60 and $600,11,18 demonstrating clearly the tremendous differences in performing neurosurgery in developing and developed countries, and by implication the overpricing and inflationary effect of the medicolegal and commercially driven environment. Fig. 2.1 Computed tomography (CT) scans of children with posthemorrhagic hydrocephalus obtained a few years after shunting. Differences in the size of the ventricles are observed. The smallest ventricles are seen in the child with (A) a differential pressure valve, the largest ventricles in the child with (B) a flow control valve. (C) The child with a valve with an antisiphon device has moderate-size ventricles. For most hydrocephalic children older than 2 years and most adults, a medium-pressure valve with an antisiphon device offers good long-term results. Adult patients are not particularly susceptible to overdrainage, so even valves without antisiphon devices offer good results. Neonates, young infants, and children in the first 2 years of life probably benefit most by a flow control valve, as they are the most likely to develop slit ventricle syndrome. Children with established slit ventricle syndrome can be difficult to manage and often require several valve trials. They are most likely to need change from a differential pressure to flow control valve and can benefit often by adjustable valves if they have developed poor compliance and are very sensitive to small changes of pressure. Young infants with very big ventricles and a small cortical mantle are particularly susceptible to overdrainage, collapse of the cortical parenchyma, and development of subdural hematomas; thus, they should have high resistance valves (high pressure + anti-siphon or flow control or adjustable set at high pressure). Another controversy that has arisen in the last few years is whether adjustable pressure valves are superior to fixed pressure valves. This debate has evolved for two main reasons: the shunt manufacturers decided to promote the marketing of adjustable valves as the concept seemed to gain market acceptance, and NPH has become a more prominent focus in comparison to 10 or 20 years ago, with the view, promoted by shunt manufacturers, not proven by robust clinical trials, that adjustable shunts are more suitable to patients with NPH. Critical assessment of the published evidence does not support the idea of any superiority of adjustable pressure valves. Before embarking on comparisons between these two types of differential pressure valves, it is important to remember that for at least 3 decades children and adults worldwide have been treated with fixed pressure shunts with very good results. Fixed pressure shunts have a consolidated profile of complications,19–23 which makes management of patients predictable and, to a large extent, easy. On average, a newly implanted fixed pressure valve, whether differential pressure with or without antisiphon device or a flow-controlled valve, has a 1-year survival rate of 70%, a 5-year survival rate of 55 to 60%, a 10-year survival rate of 20%, and an immediate postimplantation complication rate (including infection) of ~10%.22 Symptomatic slit ventricle is a clinical problem often difficult to solve, but thankfully it is not that common, seen probably in < 20% of shunted children, and rarely in adults. Millions of children worldwide have benefited from fixed pressure shunts, completed school, completed higher education of some kind, and enjoy a near-normal life. This was not possible before the invention of the differential pressure valve. Several clinical studies have established the use of adjustable valves in both children and adults.24–29 All studies declared that adjustable valves have the same rate of complications as fixed pressure valves, not less. A randomized, controlled study by Pollack et al between the Codman Hakim programmable valve and fixed pressure valves was not designed to demonstrate if the ability of pressure change confers any clinical advantage; it was only designed to show if the two types of valves had similar complication rates.25 The main advantage of adjustable valves is the facility to change the opening pressure of the valves without resorting to an operation. Most studies with large patient numbers have demonstrated that 50 to 60% of patients required at least one adjustment of the pressure setting. Nevertheless, in all studies it is clear that there is no well-defined protocol for changing the opening pressure. Some surgeons use adjustable pressure valves in all cases,27–29 whereas others use them selectively for patients who may require pressure change (e.g., in slit ventricle syndrome).24 Another shortcoming is the lack of recommendations regarding the startup setting and the criteria for changing the pressure setting. It seems that pressure change has been done at surgeons’ discretion or interpretation of the symptoms or because of a lack of improvement on the part of patients. An exception is patients who develop postshunting subdural hematomas who invariably have the valve pressure setting increased. Even in these patients, additional drainage of the subdurals via a burr hole has been required in a significant percentage.25,26 Although no clear consensus exists, it appears that adjustable valves may be useful in children who suffer from slit ventricle syndrome and have tried other valves and in adults with NPH. They may be useful in other circumstances where incremental change of intraventricular pressure may be beneficial, for example, in neonates with very large ventricles with a thin cerebral mantle, where there is a significant risk of subdural collections following shunting (Fig. 2.2), and in children with large convexity arachnoid cysts where fixed pressure valves in a cystoperitoneal shunt can cause overdrainage.30 Fig. 2.2 CT scans of a neonate with hydrocephalus and an abnormally thin cerebral mantle. (A) A few days after insertion of a valve with an antisiphon device, large subdural collections developed. (B) The original valve was removed, and an adjustable valve was inserted and gradually increased in opening pressure, until the subdural collections were absorbed. Invariably, all adjustable pressure valves have a significantly higher cost than standard fixed pressure valves, on the order of 30 to 50%, depending on local pricing policies. Some proponents of the routine use of adjustable valves for all patients and all ages have tried to argue that the excess cost is being offset by the long-term saving that the option of pressure change incurs. That economic argument has not been proven yet. A speculative study has been performed,10 but it has no real value, as it attempted to calculate the projected economical saving rather than compare prospectively actual cost efficiencies in implanted patients. There is no prospective study that compares the cost of adjustable versus nonadjustable valves, and it would be impossible to carry out such a study, having in mind the problems that patients with hydrocephalus, especially children, have over several years (repeat admissions for possible obstructions, repeat scans for investigations of headaches, doctors’ differing attitudes to symptoms, etc.). Certainly, the current evidence in the literature does not justify the routine use of adjustable valves in all patients and all ages. Fixed pressure shunts have an established profile of success, complication, and long-term failure rates. They have proven their value with time. Many new shunt designs have appeared in the past 20 years, but none have managed to make a dramatic impact on the success, complication, and long-term failure rates. Adjustable pressure shunts may be good for specific types of patients, such as children with symptomatic slit ventricle syndrome or adults with NPH. The routine use of adjustable pressure shunts has not been proven justifiable yet. The development of the first valves for CSF diversion shunts occurred in the 1950s. Because ventriculoatrial shunts were common at the time, Matson and Alexander realized that a one-way valve was necessary to prevent reflux of blood from the atrium of the heart into the ventricular system.31 Several basic valve designs were developed, including ball-in-cone, diaphragm, and slit valves.32,33 During the ensuing decades, as ventriculoperitoneal shunts became preferred, a further benefit of shunt valves was recognized, the shunt valve’s ability to regulate CSF flow. It is this capability that led to the next innovation in valve technology: adjustable shunt valves. The earliest adjustable designs date to the 1970s and 1980s.31 Adjustable shunt valves provide a way to change the amount of CSF drainage without the need for further surgery. In the early 20th century, insightful experiments by Dandy and Blackfan, among others, demonstrated the nature of obstructive and communicating hydrocephalus.34 As was later observed, all hydrocephalus is obstructive in point of fact, but when the obstruction is at the level of the subarachnoid space or arachnoid granulations, it is commonly referred to as communicating.35 The normal in vivo situation is not well mimicked by a CSF shunt. It has been observed that normal CSF flow is pulsatile, whereas the flow in a ventriculoperitoneal shunt tube is largely continuous and subject to gravitational forces as well as ICP.36 Differential pressure valves then compensate for these forces by providing a fixed opening pressure so that continuous drainage does not occur. Below the opening pressure, CSF flow decreases to negligible amounts, whereas above the opening pressure, the pressure head behind the valve drives the flow. What determines the right opening pressure for a given patient? The shunted patient is an artificial situation in which slight changes in position may potentially dramatically alter the pressure difference across the valve. Brain physiology must adapt to this foreign situation, but we have no way of telling what the ideal opening pressure may be a priori. Some patients may do well with their initial fixed differential pressure valve. Nonetheless, in a significant proportion of patients, the initial fixed differential pressure valve may cause problems. These problems, which will be considered more fully later, are related to either over- or underdrainage. Briefly, we will review the basic facts of shunt valve design. Fixed differential pressure valves come in a variety styles. The original Holter valve uses two tubes with slits to both produce one-way flow and create a fixed resistance, or opening pressure. Valves of a similar nature include the Phoenix valves (Vygon Neuro, Valley Forge, Pennsylvania). Another variation on the slit valve is the miter valve, such as the Mishler valve (Integra). Other fixed valves use a ball and spring to put resistance across the small opening inside the valve chamber. The Hakim valve (Codman & Shurtleff) is a ball-and-spring type well known for its durability. The GAV and Paedi-GAV (Aesculap Inc., Center Valley, Pennsylvania) combine a ball-and-spring design with an integral antisiphon device, which uses weighted balls to compensate for the effects of patient position. Several other valve designs, for example, the PS Medical valves (Medtronic), use a diaphragm to regulate flow across the valve opening. Flow control valves represent a different concept in valve design. These valves attempt to hold flow constant across a broad range of pressures. The well-known valves in this category are the Orbis-Sigma valve and the OSV II (Integra). Fluid pressure drives a small conical piston so that, when pressure is higher, the opening for fluid flow is smaller. This allows relatively even flow over a wide range of pressures. The shunt design trial of the 1990s failed to show any benefit to this design over fixed pressure valves in terms of failure rate or patient outcome.37 The concept of a noninvasively adjustable valve dates to at least the 1970s.31 Several adjustable shunt valves are on the market today. The Codman Hakim programmable valve uses a ratchet mechanism to control the resistance of the spring mechanism that controls a ball across the valve opening. The ratchet mechanism is adjustable by an external magnet using an electrically operated programming unit. The standard valve is adjustable from an opening pressure of 30 to 200 mm H20. After programming, the setting of the valve can be checked using a skull radiograph by comparing the position of a movable radiopaque marker with fixed markers on the valve. The valve is also available with a separate antisiphon component. Because this valve and other adjustable valves rely on a magnet to program the opening pressure, rechecking the setting is suggested following magnetic resonance imaging (MRI) scans. Fig. 2.3 Graphical representation of adjustable shunt valve function. Each line represents the pressure versus flow curve for a given setting of the adjustable valve. As the valve is set to higher opening pressures, less flow occurs at any given pressure. Similarly, the Medtronic PS Medical Strata and Strata II valves use a magnet to noninvasively adjust the tension on a spring, which seats a ball inside a conical opening. Increasing tension on the spring increases valve resistance. The setting of the valve can be obtained without radiographs using a handheld indicator tool, which comes in both a manual and an electronic version. This valve typically comes with an integral antisiphon device; however, the valve is also available without the antisiphon component. The valve is adjustable to five different levels of resistance or “performance” levels, from 0.5 to 2.5. The Sophy valve, manufactured by Sophysa (Orsay, France) and first introduced in 1985, is the oldest commercially available adjustable valve. It also uses a magnetically movable fulcrum to adjust the tension on a synthetic ruby ball that is seated within the flow pathway. The Polaris valve, which is marketed as MRI-compatible, is an updated version of the Sophy adjustable valve. Aesculap markets the pro-GAV adjustable valve based on the fixed GAV shunt design (Miethke). This valve is housed in a titanium metal casing unlike the plastic coverings of the other valves. Similar to the others, tension on a ball is adjusted with an external magnet and read with an indicator tool. The design also incorporates an antisiphon device. In summary, several adjustable shunt valves are available, each with minor variations. There are no clinical studies that evaluate one design over another. Surgeon preference regarding such subtleties as size, ease of use, availability, and familiarity largely drives the decision over which one of the adjustable valves to use. Ventriculoperitoneal (or ventriculoatrial or ventriculopleural) shunts are conceptually simple devices, and the clinical uses of adjustable shunt valves are straightforward. Adjusting the valve opening pressure lower results in less resistance to flow, and higher CSF flow rates are obtained. Similarly, higher opening pressures result in higher resistances and less flow under similar conditions. When plotted as a pressure versus flow curve, the different valve settings typically give a series of parallel curves (Fig. 2.3). Each line in Fig. 2.3 represents the performance of the valve at a different resistance setting. This adjustable resistance feature is useful in several clinical situations. Because the correct opening pressure for a given patient is initially unknown, a starting opening pressure must be estimated. A typical dilemma occurs with patients with large ventricles. These types of pediatric patients are not uncommon in centers where treatment for hydrocephalus is delayed. One must weigh the desire to provide high CSF flow to reduce the size of the ventricles against the risk of causing a subdural hematoma by rapid collapse of the ventricles. Numerous publications have documented the utility of having an adjustable shunt valve in this situation. By raising the opening pressure of the valve, an incipient subdural hematoma can be treated noninvasively without the need for valve revision or evacuation of the subdural fluid (Fig. 2.4). A second type of patient in which an adjustable valve can be useful is the patient with slit ventricles. Here shunt overdrainage leads to an abnormally small amount of CSF within the ventricular system. Symptoms include chronic headaches and frequent shunt malfunctions. Gradual elevation of the opening pressure can allow for reexpansion of the ventricles.38 This reexpansion may also lead to fewer proximal malfunctions, as the ventricular walls are not coapted. Noninvasive manipulation of the opening pressure makes this procedure much more tolerable. Fig. 2.4 Clinical examples of adjustable shunt valve use. (A) This patient presented at 16 months of age with severe communicating hydrocephalus and a head circumference of 61 cm. (B) The patient underwent placement of a right occipital ventriculoperitoneal shunt with an adjustable shunt valve. (C) Over the course of several months, large bilateral subdural hematomas developed. (D) The patient’s shunt valve was reset to a higher resistance with gradual resolution of the chronic subdural collections. (E) Long-term follow-up shows complete resolution of the subdural hematomas. The patient continued to improve neurologically after placement of the shunt. Occasionally, the neurosurgeon will encounter situations where shunt underdrainage is a problem. In neonates, in particular, underdrainage is often accompanied by accumulation of subgaleal CSF collections. These collections can put the healing incision at risk. After a shunt obstruction has been ruled out, this situation can sometimes be treated noninvasively by decreasing the opening pressure of the valve. Numerous retrospective studies have documented the effectiveness of adjustable shunt valves for various purposes. In 2005 Kestle et al published a prospective multi-center study of the Strata valve documenting its efficacy and safety.39 The first-year revision rate for these patients was similar to patients with fixed pressure valves. Nevertheless, the adjustable property was found to be useful in a large number of patients. Similar favorable results with other types of adjustable valves have been found by others.40–42
Nonadjustable (Fixed Pressure) Shunt Valves
Evolution of Valve Designs
Adjustable Valves
Controversies Regarding Shunt Valves
Fixed Pressure versus Adjustable Pressure Valves
Conclusion
Programmable Shunts
History
CSF Dynamics
Valve Technology
Clinical Uses
Clinical Studies and Evidence
Efficacy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree