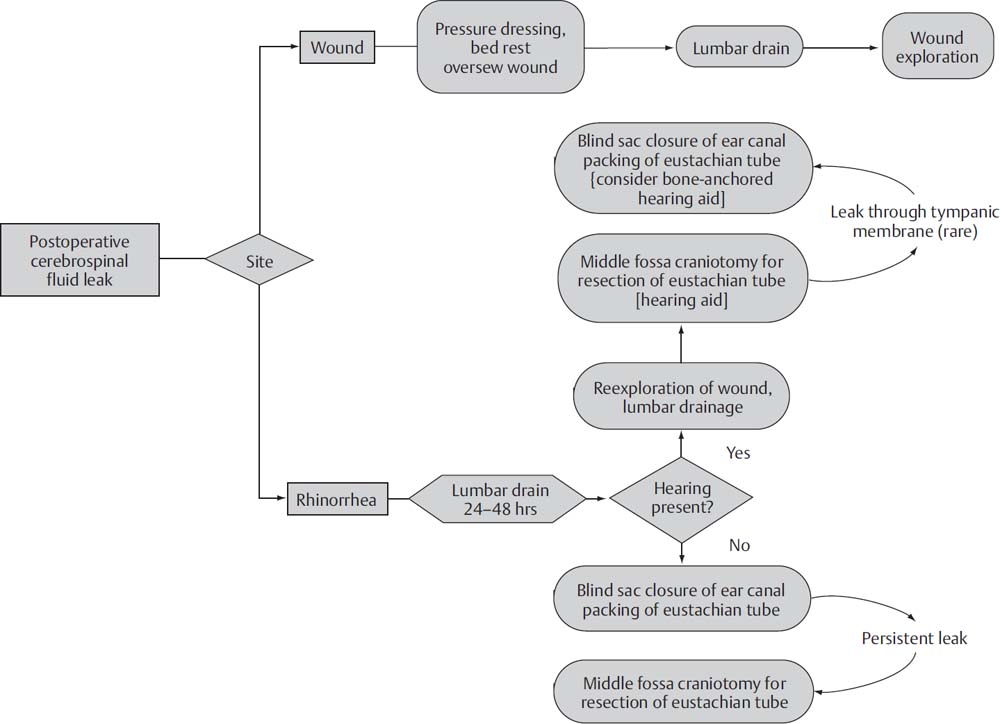
15 The treatment of vestibular schwannomas (VSs) and other skull base lesions has evolved quite remarkably since the first surgery for VS was described by Ballance in 1895. Whereas the reduction in mortality was the focus of surgical advancement in the first half of the 20th century, the reduction in morbidity associated with cranial neuropathies has been the goal since that time. This has largely been accomplished with the introduction of the microscope to neurotology, intraoperative cranial nerve monitoring, and advances in neuroanesthesia. Despite the current low incidence of complications of neurotologic surgery, an understanding of the pathogenesis, predisposing contributing factors, and management of these complications is critical. Prevention of complications begins with a careful preoperative evaluation of the patient. A careful neurotologic examination will reveal preexisting neurologic deficits such as facial weakness or numbness, signs of cerebellar compression, and hydrocephalus. Preoperative imaging aids in confirming not only the tumor type and size, but also the relationship of the tumor to surrounding neurovascular structures. Tumor size and proximity to critical structures is directly related to anticipated complications and cranial nerve deficits. An appropriate medical evaluation of the patient is also mandatory. Medical comorbidities should be identified and controlled as well as possible prior to subjecting the patient to a neurotologic procedure. At our institution, we include in the health care team an internist experienced with the care of the neurotologic patient. This expertise has greatly augmented the perioperative care of our patients. Cerebrospinal fluid (CSF) leak is one of the most common complication of acoustic neuroma surgery, with a reported incidence of 2 to 30%. This problem persists despite advancement in acoustic tumor surgical techniques, and contributes to prolonged hospital stays and postoperative meningitis. Our current rate of CSF leak at the House Clinic is 4.3%, which compares favorably with the 9.4% incidence published in the past. Only one third of patients who experience a postoperative CSF leak require an additional procedure for control of the leak. The remaining patients are managed conservatively. We attribute this low incidence to our current intraoperative closure practice, as outlined below. In any surgical approach to acoustic neuroma, the pneumatized air cell system of the temporal bone is entered, and a potential communication between the temporal bone and the subarachnoid space can persist, resulting in leakage of CSF through the skin incision or the eustachian tube (presenting as rhinorrhea). Despite differences in technique, no significant difference in the rate of CSF leaks among the translabyrinthine, retrosigmoid, and middle fossa approaches to acoustic tumor surgery has been shown. Closure of a translabyrinthine craniotomy involves obliteration of the partial dural defect with fat harvested from the lower abdomen. The fat is cut into strips and soaked in bacitracin. The dura is closed along the posterior fossa incision, and fat strips are tightly packed into the dural opening and the internal auditory canal (IAC), extending 2 cm into the cerebellopontine angle. Additional fat is packed in the mastoid defect. The incus is removed and the eustachian tube is filled with Surgicel mixed with bone wax, followed by muscle. The middle ear space is also obliterated with muscle. Alternatively, the incus may be left in place and the antrum packed with muscle. A titanium cranioplasty mesh is inserted over the mastoid cavity to better secure the fat grafts. Any air cells exposed during the procedure are sealed with bone wax. The wound is closed in layers and a pressure dressing is applied. Following tumor removal in a retrosigmoid approach, we prefer to use bone wax to seal all exposed air cells. The petrosal defect created after opening of the IAC is packed with abdominal fat and secured with dural suture or fibrin glue. The wound is then closed in layers, and a compressive dressing is applied. In a middle cranial fossa approach, all exposed air cells are waxed and obliterated with abdominal fat, and the IAC is filled with abdominal fat. Air cells or dehiscences in the floor of the middle cranial fossa are waxed or covered with fascia. Following release of retraction of the temporal lobe, any dural defects are repaired with suture or fat graft. The craniotomy bone flap is replaced, the wound closed, and a pressure dressing is applied. Management of the patient with a postoperative CSF leak must be tailored to the hearing status of the patient, as well as the site of the fistula. A variety of options for treatment exist, ranging from conservative measures such as bed rest and lumbar drainage to surgical revision. Below is a logical algorithm used at the House Clinic for management of postoperative CSF leaks (Fig. 15.1). Various protocols for conservative management of CSF leaks have been described. A recent meta-analysis of 25 studies concluded that for incisional leaks, conservative management, including wound resuturing, pressure dressings, bed rest, and head elevation, is indicated for the first 48 hours. Should this fail to control the leak, continuous lumbar drainage may be performed for 2 to 5 days. Surgical reexploration is indicated for persistent leaks. Cases of rhinorrhea do not respond well to conservative management, and thus are treated initially with 2 to 5 days of lumbar drainage, followed by surgical revision for recalcitrant leaks. We prefer early surgical intervention to prevent the development of meningitis seen with prolonged leaks. Surgical management of recalcitrant CSF leaks first involves exploration of the craniotomy cavity. Abdominal fat used during the primary surgery is removed and repacked. Any air cell tracts that are discovered are sealed with bone wax and abdominal fat. In patients without serviceable hearing, the middle ear is also obliterated with abdominal fat, and the eustachian tube orifice is sealed with bone wax and a muscle graft (Fig. 15.2). The ear canal is closed in a blind-sac fashion. This is done so that the anterior bony annulus may be removed to expose the introitus of the eustachian tube. It is important to carefully examine all possible dural sources of CSF leakage as well as potential air cell routes to the eustachian tube. Revision surgery is nearly 100% effective in treating CSF rhinorrhea when a correctable defect is observed. Fig. 15.1 House Clinic algorithm for the management of cerebrospinal fluid (CSF) leak after craniotomy. From Friedman RA, Cullen RD, Ulis J, Brackmann DE. Management of cerebrospinal fluid leaks after acoustic tumor removal. Neurosurgery 2007;61(3 Suppl): 35–39. Reprinted by permission.
Complications of Neurotologic Surgery
 Preoperative Workup
Preoperative Workup
 Complications
Complications
Cerebrospinal Fluid Leak
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neupsy Key
Fastest Neupsy Insight Engine
