Computer-Assisted Data Collection and Analysis
Pierre LeVan
Jean Gotman
Introduction
When evaluating patients with epilepsy, it is often important to obtain a thorough documentation of ictal and interictal electroencephalographic (EEG) patterns, preferably in conjunction with behavioral patterns. This is critical in the differential diagnosis of epilepsy and in the determination of the seizure type so that adequate medical treatment can be administered. An EEG investigation can also provide significant information for the evaluation of candidates for epilepsy surgery. Long-term monitoring sessions are commonly performed during which the EEG and the patient’s behavior are observed and recorded continuously. During this time, all spikes and seizures are recorded, observers can interact with the patient during seizures, and EEG and clinical correlations can be performed fully. In some cases, a patient might be monitored during several days or even a few weeks. This might be necessary if a patient’s seizures occur sporadically or if a patient suffers from multiple seizure types that need to be properly characterized. Prolonged monitoring might also be required to measure the effect of various antiepileptic drugs (AEDs). Continuous observation and recording of the EEG, typically with 32 to 64 channels, and of behavior during several days or weeks is an immense task. Continuous monitoring is expensive due to the requirements for considerable personnel and equipment during a prolonged period. If sufficient personnel are not available for constant observation, the following questions arise: Should the entire recording be reviewed, which is a very time-consuming task, or should a selective review be performed? If a selective review is performed, how is the selection to be made? Or should the recording be selective, including only the events of interest? Can these tests be performed efficiently and reliably in an outpatient environment?
It has become clear in recent years that computers can be extremely helpful in making the procedure of long-term epilepsy monitoring less tedious and less expensive. They can also assist in the review and analysis of the EEG of epileptic patients, and they can be used for data archiving. Behavioral video recording also can be performed with the assistance of computers. The first part of this chapter considers how computer-based recordings, particularly automatic methods for detection of spikes and seizures, can facilitate long-term epilepsy monitoring in the hospital and at home. The second part discusses the role of computers in EEG review, analysis, and archiving.
Computer-Assisted Long-Term Electroencephalographic Monitoring
In the past, a continuous recording lasting 12 to 24 hours, with the typical 16 channels, could be performed only with a traditional EEG machine and paper or with magnetic tapes running at very low speed. Both media have a limited channel capacity; paper in such a quantity is obviously cumbersome and expensive, and tapes have the disadvantage of being accessible only sequentially. Today, the capacity of computer disks is such that it is simple to perform a continuous 32-channel, 24-hour recording on a standard computer. A computer-based recording allows a flexible review procedure, with random access to any part of the recording and the availability of numerous methods of data manipulation and analysis (see the later section on analysis).
In most 24-hour recordings, 95% to 99% of the recording offers little useful information for the evaluation of an epileptic disorder. Automatic detection methods, despite their imperfections, can facilitate the recording, and thus the review, of valuable information. The recordings of seizures and of interictal spikes are discussed separately. Several reviews of automatic event detection in epilepsy and applications of computer methods to the analysis of the EEG in epilepsy have been published in the last few years.30,40,41,52,75,122
Recording Seizures
In the context of epilepsy monitoring, the term seizure must be defined. A behavioral seizure can be defined as the clinical manifestation of an epileptic seizure as perceived by the patient, seen by an observer, or recorded on video. The electrographic or EEG seizure can be defined as an abnormal, paroxysmal EEG pattern. In a large fraction of cases, behavioral and electrographic seizures can be observed simultaneously; this is why it has been possible to characterize the EEG changes specific to seizures. There are many cases, however, in which a discrepancy can be observed between behavior and EEG. Two types of discrepancy are possible:
A behavioral seizure can be present in the absence of visible EEG changes. If it is assumed that the seizure is indeed epileptic (the means of differentiating epileptic from nonepileptic seizures are not discussed here), then abnormal EEG activity is actually present somewhere in the brain. It is simply not available to the particular method of observation, that is, to the particular arrangement and location of electrodes. The discharge might, for instance, be limited to mesial frontal regions, whereas electrodes might have been placed only on the scalp or only intracerebrally in the temporal lobes. For the purpose of epilepsy monitoring, it must be clear that such a seizure will certainly be missed by observation, review, or computer analysis of the EEG alone.
An EEG seizure can be present in the absence of clinical manifestations. This is commonly referred to as a purely electrographic seizure or a subclinical seizure. It was argued in the previous discussion that an absence of EEG discharges can be a pitfall of the method of measurement
(a discharge can be present but not be discernible to the electrodes). It similarly can be argued that the absence of a behavioral seizure may be a pitfall of the method of observation: If a patient is lying in bed watching television, unresponsiveness, inability to speak or understand, and minor automatism or loss of muscle tone cannot be observed unless active testing is performed. Even giving correct responses to many questions does not exclude clinical signs; seizures involving only memory have been observed.100 In other words, the presence of clinical signs is a function of the method of questioning—hence the importance of recording all seizures, with and without overt clinical signs, and of observing them as precisely as possible. The importance of subclinical seizures has been discussed with respect to evaluation for epilepsy surgery.115 It will be shown later how automatic seizure detection and seizure warning systems can be helpful in this situation.
It is thus obvious that all types of seizures, with or without clinical and EEG manifestations, ideally should be recorded.
Continuous Recording and Review
As indicated earlier, it is possible to perform a continuous recording on a computer disk for a period of 24 hours. This represents a large amount of EEG to be reviewed, but computer review on a high-resolution screen can be quite fast; 32 channels of EEG can be presented 10 to 20 times faster than real time, resulting in a review lasting 1 to 2 hours. However, such a fast review can result in small seizures being missed, particularly when the number of channels goes beyond 12 or 16.101 The period of 1 to 2 hours just mentioned does not include the time required for the analysis of seizures or other potentially interesting events. Therefore, the full review of a 24-hour recording is quite time consuming and strenuous. Alternative strategies have been developed by many institutions.
These strategies consist mainly in using a partial review with an attempt to select the most relevant sections. The most important sections are, of course, those during which the patient or an observer noted a behavioral seizure. In addition, randomly selected sections are often reviewed as well. As discussed previously, this entails the significant risk that seizures of which the patient is unaware and that are not observed will be almost systematically missed. This might be an acceptable compromise if the patient is under close observation, but it could be quite dangerous otherwise.
Automatic Detection of Seizure Patterns
Some methods of seizure detection are based on behavioral manifestations of seizures; mechanical sensors under a mattress can detect the strong rhythmic movements of generalized tonic–clonic seizures. The limitations of this kind of method are obvious—many seizures do not include such strong movements. This discussion concentrates on seizure detection methods based on EEG analysis. As indicated earlier, some seizures do not have EEG changes, and EEG-based methods cannot be expected to detect them. There are also seizures that have mild or nonspecific changes, such as brief desynchronization, or groups of theta or delta waves.4 These EEG patterns are only interpreted as being abnormal in the context of a concomitant clinical event. This makes it very difficult for an automated seizure detection system to differentiate these seizure discharges from normal EEG patterns because the computer does not take into account clinical information when analyzing the EEG. There are thus limitations to seizure detection by EEG; fortunately, these limitations are not very significant, because the vast majority of seizures are associated with clear and relatively specific EEG changes.
The problem of seizure detection is inherently difficult because seizure activity can comprise a variety of morphologies; unlike spikes, which have relatively well-defined characteristics, seizures can include patterns such as low-amplitude desynchronization, polyspike activity, rhythmic waves at a wide variety of frequencies and amplitudes, and spikes and waves.13 In extracranial recordings, seizures are often obscured by electromyographic, movement, and eye blink artifacts. From the point of view of pattern recognition, the problem is therefore complex. We first discuss the methods of detection, and then address the complex issue of establishing these methods in a practical clinical context.
Methods of Seizure Detection
Early methods for seizure detection relied on relatively simple measurements of amplitude. Because an increase in amplitude is fairly common in the ictal EEG, such systems could successfully detect many seizures. Prior et al.103 described the use of their cerebral function monitor to identify generalized tonic–clonic seizures; these could be recognized on the tracing as a large increase followed by a clear decrease in EEG amplitude (the postictal depression) and by increased electromyographic activity. Such large seizures with major changes could also be identified in monkeys with experimentally induced epilepsy by a characteristic pattern on slow paper tracings (0.25 mm/s).38 Ives et al.63 described a method in which 16 channels of EEG were added, bandpass filtered, and subjected to amplitude discrimination. This technique could detect large seizure discharges but was quite insensitive. Babb et al.8 implemented an electronic circuit for the detection of seizures in recordings from intracerebral electrodes. A seizure was recognized when a rapid succession of large-amplitude spikes, lasting at least 5 seconds, was found.
More sophisticated systems computed several features of the EEG to recognize seizure activity. Murro et al.93 described a method based on spectral parameters and discriminant analysis. Gotman37,43 presented a computer method that attempted to recognize a wide variety of seizure morphologies, identifying patterns that might represent seizure activity for later examination by traditional visual inspection. The method was therefore designed to be as sensitive as possible. False detections, as long as they were not extremely frequent, were not detrimental because they could be removed in an ensuing visual review of the detected events. Observation of numerous seizures led to the conclusion that most seizures, at some time during their development, included activity that was paroxysmal compared with the background (the paroxysm could consist of increased amplitude or increased frequency); activity also had to be rhythmic (with frequencies varying from 3 to 20 cycles per second) and relatively sustained in duration (lasting several seconds).
Measurements of these characteristics were obtained by breaking down the EEG into half-waves; for every 2-second epoch, the average amplitude of the half-waves relative to that of the background (indicating whether an epoch was paroxysmal), the average duration of the half-waves (indicating frequency), and the coefficient of variation of half-wave duration (indicating the regularity of duration or the rhythmicity) were measured. The background was constantly updated and included EEG sections before and after the active epoch. Comparing the amplitude, frequency, and rhythmicity measurements of the background to those of the active epoch allowed definition of a decision tree for the detection of seizure activity. It is often forgotten that it is not necessary to detect the onset of a seizure; a detection occurring at any time during the course of the seizure is sufficient because the computer simply marks the time of the detection and the interpreter can page back to determine the exact time of seizure onset. This method of seizure detection has been made available commercially and is
in widespread use throughout the world. It has also been used to monitor experimental animals.12,88
in widespread use throughout the world. It has also been used to monitor experimental animals.12,88
Harding57 presented a method specifically designed for intracerebral recordings based on the detection of a repetitive spiking pattern as well as of possible flattening at seizure onset. This method was implemented online and was subject to an extensive evaluation (see later discussion). Another approach for seizure detection was proposed by Schindler et al.112 They simulated neuronal cells processing each EEG channel and used leaky integrators to assess increases in amplitude and slope over a certain time.
Artificial neural networks (ANNs) have found broad applications in many areas of pattern recognition, including seizure detection. Jando et al.64 showed the utility of neural networks for detecting bursts of spike-and-wave in experimental models of epilepsy. Since then, several methods have been presented involving the use of various EEG features as inputs to an ANN.6,32,116,120 By being presented with a sufficiently large number of training examples comprising seizure and nonseizure data, the ANN can learn to differentiate seizures from normal EEGs. A representative set of features characterizing the EEG needs to be specified in advance (e.g., amplitude, rhythmicity, dominant frequency, and so on), but it is not necessary explicitly to describe the seizure patterns. The ANN automatically determines which combinations of features are characteristic of seizures by adapting its internal structure to reflect the training data. The performance of ANN methods depends on the selection of an appropriate set of features and on the use of a sufficiently large training set containing a wide variety of seizure patterns. It is also necessary to specify a priori the structure of the ANN; large networks can handle complex classification tasks but may risk overfitting the training data and thus be unable to generalize to other types of seizures. It is also very difficult to investigate the operation of a large ANN; the network essentially acts as a black box that outputs classification values depending on the features it receives as inputs. To circumvent this issue, Wilson et al.124 presented an algorithm based on a general set of rules in which each individual rule was implemented using a small ANN. By using several simple rules, it became easier to analyze the operation of the system.
Recent developments in automatic detection have demonstrated that performance can be improved significantly by incorporating a wide context in detection algorithms. Rather than defining the event only locally (e.g., comparing the characteristics of a 5-second epoch in one channel with the 30 seconds that precede it), it is important to include measurements of the spatial context (activity in other channels) and the temporal context (state of the subject—awake, stages of sleep, previously recorded events). Qu and Gotman104 used this philosophy to greatly reduce the false detection rate by allowing the method to remember the patterns that caused frequent false detections in each subject. Klatchko et al.68 presented a method for spatial and temporal clustering of elementary detections initially made on individual channels and epochs, which resulted in a more global representation of seizures. This reduced the number of false detections by only asserting the occurrence of a seizure if there were a minimum number of elementary detections adjacent in space and time. The system of Khan and Gotman67 was based on wavelet decomposition to detect seizures in the intracerebral EEG. Wavelet methods allow the analysis of the temporal evolution of the frequencies of a signal; they are thus well suited for the identification of paroxysmal rhythmic activity, which often characterizes seizures. However, intracerebral EEGs also frequently feature rhythmic bursts associated with normal background activity. Therefore, Khan and Gotman’s algorithm automatically adapted to the background of each patient by deactivating the detection of paroxysmal activity occurring consistently in the same channels and at the same frequencies. This resulted in an important decrease in the number of false detections (see later discussion).
Methods of Seizure Warning
Seizure detection systems can significantly reduce the load of EEG interpretation during long-term monitoring by requiring only the review of the detected events rather than the entire EEG record. Another purpose of seizure detection could be to warn the medical personnel, patient, or relatives that a seizure is starting, which could be particularly useful if the electrographic onset of the seizure precedes its clinical onset. In the context of long-term monitoring, a seizure warning system would allow close clinical observation early in the seizure and the taking of necessary precautions to avoid injuries that might otherwise occur in sudden unexpected seizures. It could also be useful for increasing the applicability of ictal single-photon emission computed tomography (SPECT) scans.11 A seizure warning system could also be implemented as an implantable device, and one could even conceive of rapid intervention in the form of electrical stimulation or drug injection to abort the seizure.80 The seizure monitoring systems described here were designed primarily to mark EEG records at any point during seizures so that the detected events could be reviewed at a later time. In contrast, seizure warning applications are meant to be used in real time and require detections that occur very early in the seizure. Moreover, the false detection rate should be very low because false alarms can be very disruptive in a clinical setting. Promising results were obtained by Qu and Gotman105,106; for each patient, a first recorded seizure was used as a template to train the detection system. This approach clearly results in the introduction of a bias in detection performance toward seizures similar to the template. Therefore, this system is highly patient specific and cannot warn of new or different types of seizures. It thus cannot be used for seizure monitoring purposes, but is still useful to assess the occurrence of a particular seizure type. The system was able to generate a warning within an average of 10 seconds of the electrographic seizure onset, with a relatively low false alarm rate. Shoeb et al.114 designed a system for seizure warning in scalp EEGs. By training the system with wavelet features specific to each patient, they could obtain a high sensitivity and a low rate of false detections.
Although patient-specific systems can be useful, more general methods are more practical in a clinical setting because they do not need to be retrained for each patient. The system of Osorio et al.98 used a wavelet filter followed by a median filter to detect the onset of seizures in intracerebral recordings. The best performance was achieved when the system was tuned to each patient, but good results were still obtained without this patient-specific adjustment. Saab and Gotman108 designed a system based on measurements of amplitude and rhythmicity in the wavelet decomposition of scalp EEGs. The wavelet features were then used in a Bayesian classifier to obtain the probability that a seizure had begun. Various criteria were used to automatically reject epochs containing alpha activity, electromyographic artifact, or abnormal signals due to defective electrodes; this allowed the system to ignore these sources of false detections, which are common in scalp EEG recordings. FIGURE 1 shows examples of seizures detected by the warning system. The classifier was trained using a large data set containing a wide variety of seizures; the system is thus sufficiently generic to be useful for seizure monitoring applications. For seizure warning purposes, it is also possible to further reduce the false alarm rate without significantly increasing the detection delay, by manually tuning the system for each patient.
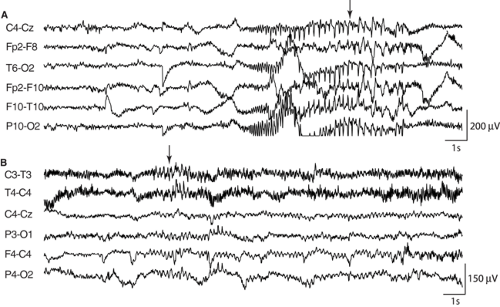 FIGURE 1. Seizures recorded by scalp electrodes that were detected automatically by the seizure warning system of Saab and Gotman.108 Arrows mark the earliest detections. A: Seizure featuring clear rhythmic spikes lasting <8 seconds. B: Subtle seizure characterized by somewhat rhythmic discharges of low amplitude. |
Whereas the system just described was specific to scalp recordings, a similar methodology was employed by Grewal and Gotman56 to process intracerebral recordings. They used spectral features in several frequency bands and integrated
measures of spatiotemporal context to detect the onset of seizures. To aid in the detection process, the harmonics of identified rhythmic signals were also examined for the presence of sustained activity. Epochs containing abnormally large amplitude signal or activity at the mains frequency of 60 Hz were automatically rejected, thus reducing the rate of false detections due to artifacts. The system was trained on a wide variety of seizures, resulting in a general detector useful for seizure monitoring, which also provided a tuning mechanism to improve its performance for use as a seizure warning system. Examples of seizures recorded by intracranial electrodes and detected by the method are shown in FIGURE 2.
measures of spatiotemporal context to detect the onset of seizures. To aid in the detection process, the harmonics of identified rhythmic signals were also examined for the presence of sustained activity. Epochs containing abnormally large amplitude signal or activity at the mains frequency of 60 Hz were automatically rejected, thus reducing the rate of false detections due to artifacts. The system was trained on a wide variety of seizures, resulting in a general detector useful for seizure monitoring, which also provided a tuning mechanism to improve its performance for use as a seizure warning system. Examples of seizures recorded by intracranial electrodes and detected by the method are shown in FIGURE 2.
The seizure warning systems described here aim to detect seizures in real time just as they are starting. On the other hand, recent studies18,61,79,87,91,95 have indicated that it is sometimes possible to detect changes in the EEG well before the actual onset of clinical and electrographic changes. These results are preliminary, but it might be possible to develop methods for seizure prediction if reliable algorithms can be designed (see Chapter 85).
Detection of Seizures in the Neonate
The detection of seizures in neonates is quite different from that in adults; discharges are often much slower (down to 0.5 Hz), seizure onset can be very gradual, seizures can last several minutes, and waveforms of seizures and of interictal background show a high level of variability. Because of this, several seizure detection systems have been developed specifically for neonates. Liu et al.84 presented a method based on the autocorrelation function for the detection of rhythmic slow patterns of any morphology. Gotman et al.47 also developed a system specifically for newborns, based on a combination of various techniques. Spectral analysis was used to detect paroxysmal bursts of rhythmic activity. Spike detection methods were also integrated in the system to detect seizures that consist of irregular spiking patterns. Very slow rhythmic discharges were detected by applying a low-pass filter to the neonatal EEG and then using the seizure detection method of Gotman43 on the filtered signal. By using a combination of several methods, the system was able to detect a wide variety of patterns occurring during neonatal seizures. Roessgen et al.107 proposed a different approach to neonatal seizure detection based on a neuronal model of EEG generation. The model takes into account the generation of seizure activity and of background activity. By fitting the model to the EEG data under investigation, it is possible to determine how much of the signal is due to seizure activity. Celka and Colditz17 presented a method based on singular spectrum analysis, also aimed specifically at seizures in infants. They used the minimum description length (MDL) principle to estimate the signal complexity, which was then used as a detection measure. Their system also implemented an autoregressive moving average (ARMA) model of the EEG as a preprocessing step to separate background activity from seizure activity. Altenburg et al.7 described a method of detecting neonatal seizures based on interchannel synchronization. They noted that the onset of seizures is often characterized by a sudden increase in synchronization. The method of Hassanpour et al.58 used time-frequency representations of the signal to detect low-frequency rhythmic activity as well as high-frequency bursts of spikes. Aarabi et al.1 designed a multistage system for seizure detection in the newborn based on a large number of features. The relevance of each feature was then analyzed, so that only the most appropriate features were selected and used in an artificial neural network to classify seizure and nonseizure EEG.
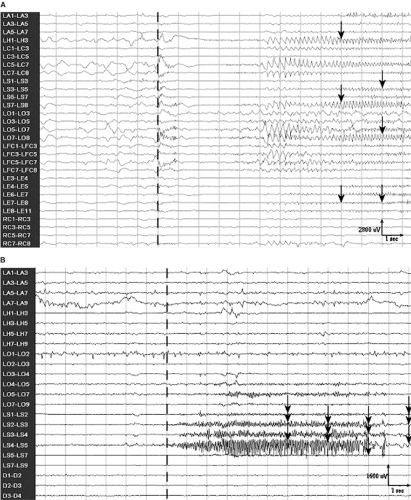 FIGURE 2. Seizures recorded with intracerebral electrodes that were detected automatically by the seizure warning system of Grewal and Gotman.56 The dashed vertical lines indicate the onset of the seizures. Arrows mark the seizure epochs that were detected by the system. A: Seizure featuring typical sustained rhythmic activity, which was detected 9 seconds after the onset. B: Seizure characterized by fast rhythmic activity. This seizure was detected only 6 seconds after its onset. |
Clinical Validation of Seizure Detection Methods
Evaluating the performance of automatic detection methods is very difficult because results may depend more on the selection
of EEGs included in the evaluation set than on the detection method itself; for instance, a seizure detection method may work well when the patient is resting with eyes closed, perform less well when eye blinks are present, and fail totally if electromyographic and movement artifacts are also included. To give a fair impression of performance, what type of EEG should be included in the evaluation? Several requirements should be met to obtain an accurate validation that corresponds to a realistic clinical setting:
of EEGs included in the evaluation set than on the detection method itself; for instance, a seizure detection method may work well when the patient is resting with eyes closed, perform less well when eye blinks are present, and fail totally if electromyographic and movement artifacts are also included. To give a fair impression of performance, what type of EEG should be included in the evaluation? Several requirements should be met to obtain an accurate validation that corresponds to a realistic clinical setting:
EEGs used in the validation should be independent of EEGs used in developing the method.
EEGs should not be preselected, especially with respect to quality. A subjective selection of the EEGs could strongly bias the results. Ideally, the records should be obtained consecutively.
A large variety of seizure patterns from several patients should be included.
A large amount of nonseizure data should also be included, to be representative of all conditions that might occur in long-term monitoring.
The performance of the seizure detection methods described earlier will be reviewed. Methods that did not use an extensive amount of independent EEG during their validation will not be considered in detail in this section. This does not mean that these methods do not perform well; in fact, most of them show very promising results. However, their validity in a realistic clinical context has not yet been established.
In evaluating the seizure detection method of Gotman,43 all efforts were made to avoid bias in data selection. EEGs were recorded from 293 consecutive monitoring sessions totaling 5,303 hours from 49 patients, most of them having medically refractory epilepsy and being considered for surgical treatment. All EEGs were included, independent of EEG patterns and technical quality. Of the 244 seizures in the validation data set, 24% were recorded by the alarm button but were missed by the computer, 35% were detected by the computer and were accompanied by an alarm button press, and 41% were detected by the computer alone. Pauri et al.101 performed an independent evaluation of the same method and came to very similar conclusions. In a third evaluation, Salinsky109 concluded that the use of automatic seizure detection made it possible to catch a large number of seizures missed by the patient and observers and to reduce significantly the length of the hospital stay. As in the previous study, approximately 20% of seizures were not detected by the computer but were detected by the patient or an observer.
It is thus clear that one cannot rely exclusively on either human observation (the alarm button) or automatic detection. Using both approaches can considerably increase the yield of long-term monitoring. In addition, the average detection rates given here reflect poorly the reality of individual patients. Some patients had most of their seizures recorded by the alarm button alone, whereas others had most of their seizures detected by the computer without an accompanying press of the alarm button. The computer can be extremely helpful when seizures go unnoticed, such as when they consist of quiet automatisms. All seizures can be detected if a nurse and an EEG technologist observe the patient and the EEG at all times. This procedure is obviously expensive and difficult to achieve—hence the importance of automatic seizure detection methods.
False-positive detection rates were around one to two false detections per hour of monitoring,43,101 increasing in some patients with intracerebral electrodes to up to four or five false detections per hour. In the majority of cases, the number of false detections is small enough not to be disruptive: False detections merely cause the EEG to be marked unnecessarily and can be removed at the time of review by visual inspection. This selective visual review is clearly preferable to reviewing the entire EEG recording without the benefit of data reduction.
The wavelet-based and context-based method of Khan and Gotman67 was evaluated on recordings from 10 consecutive patients with intracerebral electrodes, each having 20 hours of recording and at least three seizures. It was compared to the original method of Gotman.43 Sensitivity was 90% for the original method and 86% for the new method. It thus appears that the original method, even when applied exclusively to intracerebral electrodes, had an excellent performance. The new method had marginally lower sensitivity, but its main advantage was its low rate of false detection of 0.3/hour, compared to 2.4/hour for the original method.
Harding57 performed a thorough evaluation of his method of seizure detection for intracerebral recordings, also by using large amounts of unselected data—almost 1,600 hours from 40 patients. He obtained few false negatives and a very acceptable level of false positives. The results are difficult to compare directly with other methods because detection parameters were altered slightly according to the results after the first seizure was recorded. Gabor31 performed an extensive evaluation of his neural network method using 4,500 hours of scalp EEG from 65 patients. The data were not preselected, so that even low-quality EEGs were included in the analysis. The original method of Gotman43 was also evaluated using the same data set, resulting in a sensitivity of 74.4% and an average false-detection rate of 3.02/hour. The neural network method yielded an improved performance, with a sensitivity of 90.0% and a false-detection rate of 1.29/hour. Osorio et al.97 evaluated their method on recordings from 15 patients with intracerebral electrodes. Their results are not presented in the traditional fashion and cannot easily be compared to those of other methods. They indicate that 100% of the 39 seizures were detected, but they only considered seizures for which there was an alarm from the nurse or the patient, and they excluded poor-quality EEGs. Purely electrographic seizures or seizures of which the patient was not aware were not considered. In addition to the 39 detected seizures, their system yielded another 866 detections that were subsequently reviewed; 92% were then reclassified as true detections, including short electrographic bursts, leaving a low rate of false detections. Saab and Gotman108 tested their seizure warning method on an independent, unselected data set including seizure and nonseizure data from 16 patients totaling 360 hours of scalp EEG. They reported a sensitivity of 77.9%, a false-detection rate of 0.86/hour, and a median detection delay of 9.8 seconds. For use specifically as a seizure warning system, it was possible to tune the system for patients with a large number of false detections. This resulted in a false-detection rate of 0.34/hours, and the sensitivity and median detection delay were almost unchanged at 76.0% and 10 seconds, respectively. Grewal and Gotman56 tested their intracerebral seizure detection method using an independent testing data set from 19 patients totaling 389 hours of intracerebral EEG. They indicated a sensitivity of 86%, a false-detection rate of 0.47/hour, and a median delay time of 16.2 seconds. They also provided a patient-specific tuning mechanism to reduce the number of false detections for seizure warning purposes. This resulted in a false-detection rate of 0.22/hour, with a sensitivity of 89.4% and a median delay time of 17.1 seconds.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






