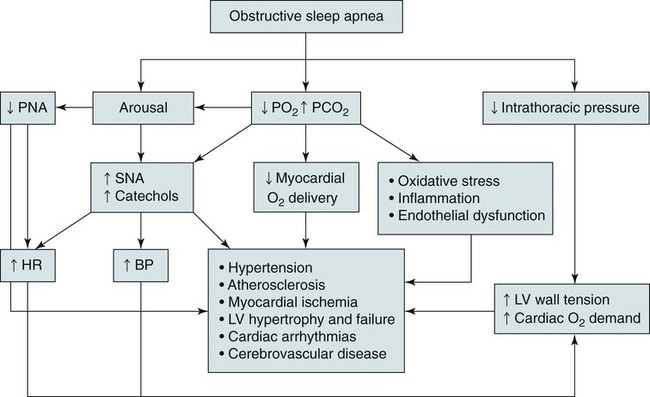
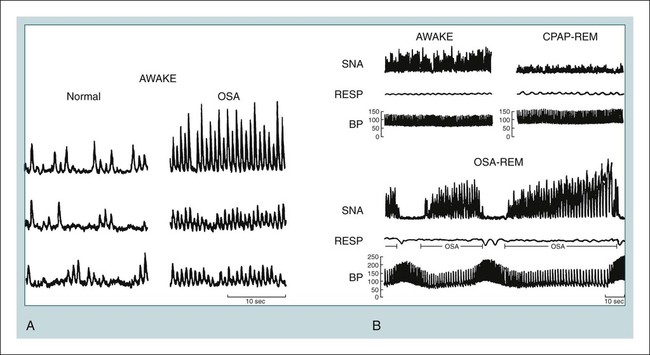
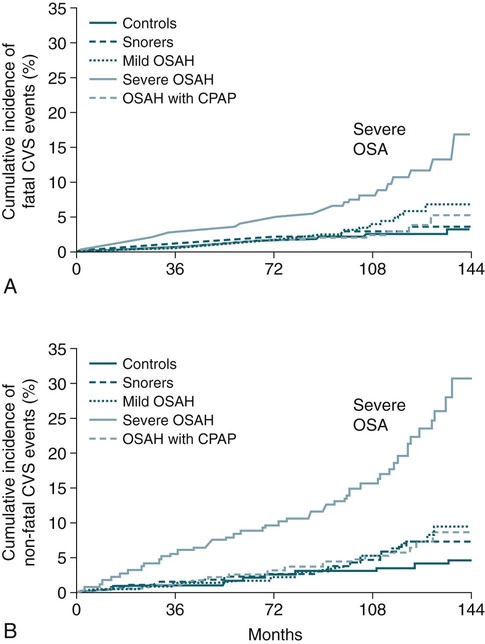
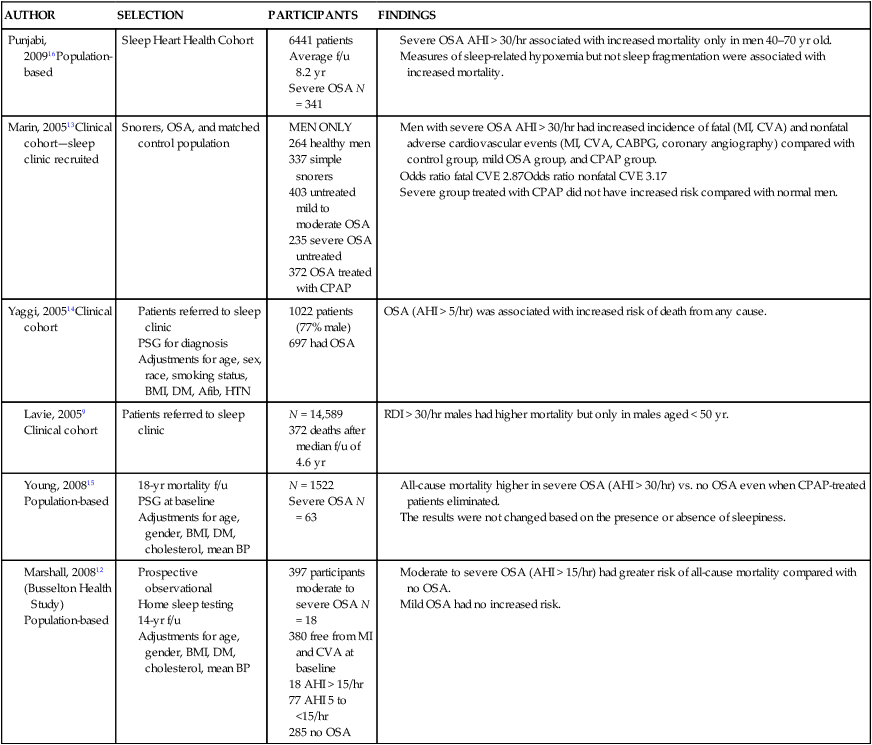
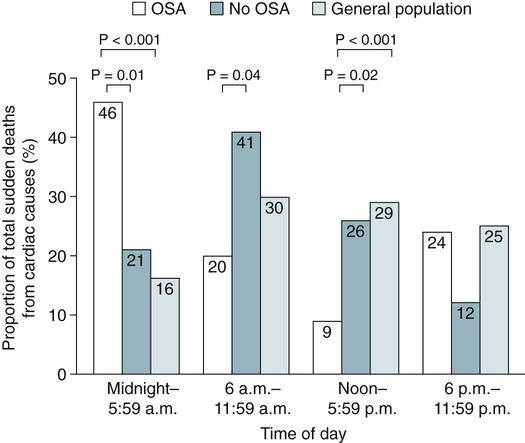
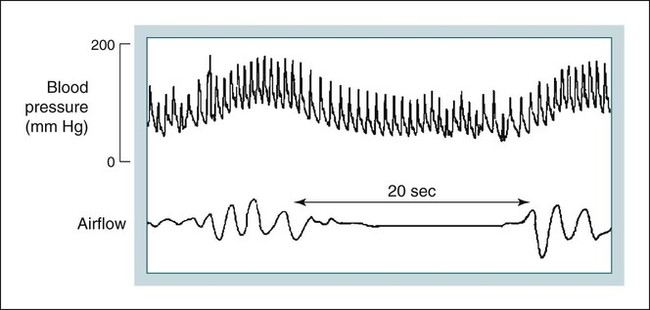
• The nocturnal consequences of OSA vary with severity. Possible changes include episodic hypercapnia, hypoxia, negative intrathoracic pressure, increases in pulmonary and systemic pressure, cyclic slowing then speeding of heart rate, and surges of sympathetic tone at event termination. Most studies show that the cardiovascular consequences are most closely related to the severity of hypoxemia. • Patients with untreated OSA often have elevated post-awakening am blood pressure, high daytime sympathetic tone, and decreased heart rate variability. • The best evidence of increased mortality in untreated OSA is for men, with moderate to severe OSA, with an approximate age range of 60 years (40–70 in other studies). • Observational studies suggest that OSA patients effectively treated with CPAP have normal survival, all other factors being equal. • The severity of daytime sleepiness increases with higher AHI but there is substantial variability. Other PSG indices such as the arousal index do not have significantly higher correlations with subjective or objective sleepiness than the AHI. • OSA patients are at increased risk for motor vehicle accidents (especially if there is a history of sleepiness at the wheel). Effective CPAP treatment can reduce the risk of motor vehicle accidents. • Patients with OSA may fail to have a fall in blood pressure with sleep (nondippers). Clinical cohorts with hypertension or resistant hypertension have a high prevalence of OSA. Although investigations differ, effective CPAP treatment likely is associated with a small decrease in 24-hour blood pressure. • OSA in the absence of lung disease or diastolic dysfunction is associated with daytime pulmonary hypertension in 20% to 40% of patients. The pulmonary hypertension is usually of a mild nature (unless other factors are present). • Untreated OSA is associated with nocturnal arrhythmia, although the incidence is low. In some cases, effective treatment will improve arrhythmias. • Untreated OSA is associated with an increased incidence of recurrence of Afib after cardioversion. • Observational studies suggest that untreated OSA is associated with a higher risk of adverse outcomes in patients with CAD. • Untreated OSA is associated with a higher incidence of stroke. In stroke patients, CPAP treatment of OSA can reduce mortality. • Untreated OSA can worsen nocturia and CPAP treatment can improve this manifestation. • Pediatric OSA has significant neurobehavioral consequences that can be improved with treatment. Considering the effects of continuous positive airway pressure (CPAP) treatment on consequences of untreated OSA, it is important to understand that observational studies are less convincing than randomized controlled trials. There is always the concern that patients who adhered to CPAP treatment are somehow more compliant with other medical therapy as well and, therefore, more likely to have better outcomes. A study by Platt and colleagues1 found CPAP users were more adherent in taking lipid-lowering medications. However, another study by Villar and coworkers2 found that patients adherent or nonadherent to CPAP did not differ in their adherence to medication. Both of these studies relied on prescriptions filled or patient report, so actual medication use could differ. There are a number of ethical and experimental considerations to designing a study to prove a benefit of CPAP treatment on cardiovascular outcomes. With the widespread knowledge of CPAP, it is difficult to design a study with effective blinding and a satisfactory control treatment (e.g., subtherapeutic CPAP).3 During normal non–rapid eye movement (NREM) sleep, there is a decrease in metabolic rate, sympathetic nerve activity, blood pressure, and heart rate, whereas vagal tone increases compared with wakefulness.4,5 OSA changes this normal pattern considerably4–6 (Fig. 17–1). Cycles of hypoxia and CO2 retention elicit changes in sympathetic and parasympathetic activity. Hypoxia and hypercapnia increase ventilatory drive and obstructed inspiratory efforts create negative intrathoracic pressure (sometimes reaching –80 cm H2O). The negative pressure increases venous return to the right side of the heart (increased right ventricular [RV] preload) and the hypoxia causes pulmonary vasoconstriction (increased RV afterload). The right ventricle may dilate and the septum bulge into the left ventricle, impairing left ventricular (LV) filling and decreasing stroke volume. Negative intrathoracic pressure increases the transmural pressure across the left ventricle walls and increases the effective afterload. The cycles of increased sympathetic tone6 (Fig. 17–2) increase systemic vascular resistance. Arousal at apnea termination is associated with a large increase in sympathetic tone and decreased vagal tone with the result that blood pressure and heart rate increase. High sympathetic tone is still present during wakefulness (see Fig. 17–2) and associated with impaired vagally mediated heart rate variability. Intermittent hypoxia causes oxygen free radical production and activates inflammatory pathways that can predispose to atherosclerosis and thrombosis. An early retrospective study by He and associates7 showed a decreased survival in untreated patients with an apnea index (AI) greater than 20/hr. Patients with an AI greater than 20/hr who were treated with tracheostomy or CPAP did not have a decreased survival. The causes of death in the patients were not documented. Lavie and coworkers8 reviewed the results on 1620 patients diagnosed with OSA between 1976 and 1988. Fifty-seven patients had died by 1990 with 53% of the deaths due to respiratory and cardiovascular causes. Excess mortality was noted in men of 30 to 50 years of age with OSA but not in patients older than 70 years of age. Lavie and colleagues9 later published results of a sleep clinic cohort (1991–2000) consisting of 14,589 patients and found that the hazard of mortality was higher in male patients with moderate and severe OSA compared with the general population. The results were significant for patients younger than 50 years of age. Another analysis of a similar cohort sought to determine the factors associated with mortality in OSA patients.10 The study found that the risk of mortality in patients with OSA was increased by the presence of co-morbidities. For patients younger than 62 years, increased mortality was associated with the presence of heart failure or diabetes mellitus. For OSA patients older than 62 years, predicators of increased mortality included chronic obstructive pulmonary disease, congestive heart failure, and diabetes mellitus.10 Older patients (age > 65 years) with mild to moderate OSA appear to be resistant to the adverse effects of OSA on mortality.11 Marshall and associates12 found an increase in all-cause mortality associated with moderate to severe OSA in a population-based study. Marin and coworkers13 studied a clinic population of men and found an increase of fatal and nonfatal cardiovascular events for severe OSA compared with normal individuals, snorers, patients with mild OSA, and patients with OSA on CPAP treatment (Fig. 17–3). Yaggi and colleagues14 published data on another cohort referred to a sleep clinic and found an increased mortality in severe OSA patients. Young and associates15 published results from the Wisconsin Sleep Cohort and found that severe OSA patients had an increased risk of all-cause mortality. The results of this study were adjusted for gender. Punjabi and coworkers16 published results on a large population cohort from the Sleep Heart Health Study. There was an increase in mortality for the severe OSA patients who were male and aged 40 to 70 years. In this study, statistical corrections for the effects of age, BMI, and gender (when appropriate) were performed. Summarizing the results, there is strong evidence for an increased mortality in untreated middle-aged male patients with severe OSA (Table 17–1). Indeed, two clinic-based population studies included only or predominantly men. Conversely, the Wisconsin Cohort data did not show a sex difference in the increased mortality associated with severe OSA. However, relatively few patients had severe OSA in this study. It seems likely that women with untreated severe OSA also have increased mortality, but the evidence for this increased mortality is not as strong as that for men. There is also the controversy about whether patients who are not sleepy have an increased risk of cardiovascular morbidity. Evidence from the Sleep Heart Health Study16 found that adverse outcomes correlated more with measures of oxygenation than with sleepiness (or impaired sleep), suggesting that intermittent hypoxemia rather than indices of sleep fragmentation is associated with increased mortality. Conversely, the Wisconsin-based cohort15 found an increase in cardiovascular mortality with severe OSA that was present irrespective of symptoms of sleepiness. The controversy continues, but it seems likely that the severity of oxygen desaturation due to OSA is the major factor associated with increased cardiovascular morbidity. TABLE 17–1 Increased Mortality and Obstructive Sleep Apnea Men with severe OSA AHI > 30/hr had increased incidence of fatal (MI, CVA) and nonfatal adverse cardiovascular events (MI, CVA, CABPG, coronary angiography) compared with control group, mild OSA group, and CPAP group. Odds ratio fatal CVE 2.87Odds ratio nonfatal CVE 3.17 Severe group treated with CPAP did not have increased risk compared with normal men. The time of sudden death appears to be different for patients with OSA. Gami and colleagues17 found that for patients with OSA, the relative risk of sudden death from cardiac causes from midnight to 6 am was 2.57 (95% confidence interval 1.87–3.52) compared with other times during the day. People with OSA have a peak in sudden death from cardiac causes during the sleeping hours, which contrasts strikingly with the nadir of sudden death from cardiac causes during this period in people without OSA and in the general population. In patients without sleep apnea, the risk of sudden death was greatest in the morning hours after awakening (Fig. 17–4).17 Excessive daytime sleepiness is a cardinal manifestation of OSA. However, the severity of this symptom is extremely variable. Chronic sleep deprivation and/or fragmentation from respiratory events is believed to be the major cause of sleepiness, although hypoxemia may also play a role. Normal individuals subjected to experimental sleep fragmentation without hypoxemia develop subjective and objective daytime sleepiness.18,19 Attempts at finding abnormalities demonstrated on polysomnography that correlate highly with subjective or objective sleepiness have not been very successful. Johns20 found the Epworth Sleepiness Scale (subjective sleepiness) to correlate with the respiratory distress index (RDI) in a group of 165 patients with OSA (r = 0.439) and slightly less with the minimum arterial oxygen saturation (SaO2; r = –0.404). Guilleminault and associates21 found no polysomnographic variable to be significantly correlated with sleepiness as assessed by the multiple sleep latency test (MSLT). Roehrs and coworkers19 analyzed data from 466 patients using multiple regression analysis and found daytime sleepiness as assessed by the MSLT had a slightly higher correlation with the respiratory arousal index (r = –0.36) than indices of hypoxemia (r = –0.34). Cheshire and colleagues found the arousal index to correlate with impaired cognitive function testing but not objective daytime sleepiness.22 Bennett and associates23 found that the AHI, cortical arousal index (central and frontal electroencephalogram [EEG]), sleep disturbance based on a neural network model, and a body movement index correlated with objective daytime sleepiness. The correlation between excessive sleepiness and the indices of sleep disturbance was only slightly higher than the correlation between sleepiness and the AHI. The body movement index (based on video recording rather than EEG) and the neural network had the highest correlation coefficients. Routine EEG analysis based on sleep staging or EEG arousals may not be sensitive enough to detect all the adverse effects of respiratory disturbance. For example, noncortical arousals from respiratory events or other causes may result in daytime sleepiness.24 It may also be possible that long periods of airflow limitation with high respiratory effort without discrete arousal could be associated with symptoms. This pattern commonly occurs in children with OSA who develop behavioral changes and impaired school performance without frequent discrete arousals.25,26 Findings from the Sleep Heart Health Study show an increase in sleepiness with higher AHI, but the relationship is weak.27 There is also evidence from several studies that hypoxemia may be a factor associated with impaired cognition in patients with OSA.28 For many patients, a number of factors other than OSA contribute to daytime sleepiness including insufficient sleep and medication side effects. There is also considerable individual variability in the ability to tolerate sleep fragmentation. Therefore, it should not be surprising that there is only a modest correlation between the AHI and subjective or objective measures. Of note, studies of heart failure patients with OSA often show a large proportion do not complain of daytime sleepiness.29 It should also be noted that effective treatment of sleepy OSA patients (e.g., CPAP) does not always abolish daytime sleepiness. The true increase in risk of patients with OSA having an automobile accident is difficult to determine. However, published studies have found a two to three times greater risk in untreated patients with OSA.30–32 Clearly, not all patients with sleep apnea are at high risk of having an auto accident. It appears that the presence of sleep apnea plus a history of a previous accident (or frequent falling asleep at the wheel) identifies a group of patients with especially high risk. The decision of whether to report a patient with OSA to a motor vehicle licensing agency is a difficult one. A balance between patient confidentiality and protection of the public is required. A committee of the American Thoracic Society has issued the following recommendations33: “In those jurisdictions in which conditions such as excessive daytime sleepiness caused by sleep apnea may be construed as reportable events, we recommend reporting to licensing bureaus if: (a) the patient has excessive daytime sleepiness, sleep apnea, and a history of a motor vehicle accident or equivalent level of clinical concern; and (b) one of the following circumstances exists: (i) the patient’s condition is untreatable or is not amenable to expeditious treatment (within 2 months of diagnosis) or (ii) the patient is not willing to accept treatment or is unwilling to restrict driving until effective treatment has been instituted.” The committee also noted that it is the physician’s responsibility to notify every patient with sleep apnea that driving when sleepy is unsafe. Some form of written documentation that the patient understands this warning is prudent. A statement concerning sleep apnea and commercial motor vehicle operators by a joint task force of the American College of Chest Physicians, the American College of Occupational and Environmental Medicine, and the National Sleep Foundation34 provides guidance concerning the evaluation, treatment, and return to work of drivers with suspected or known OSA. To date, there is no objective test that can quantify a patient’s degree of driving impairment. The use of the maintenance of wakefulness test (MWT) and MSLT were discussed in Chapter 14. The MWT is the appropriate test to assess the ability to stay awake. However, MWT results may not generalize to the real world in which sleep deprivation and other factors are operative. There have been promising attempts at developing protocols using driving simulators,35,36 but results in a simulator may not correlate with driving performance. A study of untreated OSA patients36 found an MWT mean sleep latency (MSL) less than 19 minutes was associated with impairment on a driving simulator. The same group extended this work by testing patients with OSA during actual 90-minute driving sessions on the road with a driving instructor intervening if necessary (the test car had two steering wheels).37 Inappropriate line crossing was determined by video recording. Two groups “very sleepy” with a MWT MSL less than 19 minutes and “sleepy” with a MSL 20 to 33 minutes had significantly more line crossings than controls and patients with mild sleepiness (MWT MSL 34–40 min). Studies have shown improved performance on driver simulators after CPAP treatment of OSA.38 In addition, studies have also shown that the risk of traffic accidents does appear to be reduced with nasal CPAP therapy (if patients are compliant).39,40 The evidence for a reduction in motor vehicle accidents with effective OSA treatment was recently summarized by Tregear and coworkers40 in a systematic review with meta-analysis. Normal persons and many patients with hypertension but without sleep apnea have a nocturnal fall in blood pressure. However, 20% to 40% of patients with OSA fail to have the normal nocturnal fall in systemic blood pressure (“nondippers”).41 During obstructive apnea, blood pressure tends to rise slightly and then to rise abruptly at apnea termination (Fig. 17–5) secondary to arousal from sleep and restoration of breathing associated with sympathetic activation and parasympathetic withdrawal. There is continued controversy about whether OSA can cause daytime (diurnal) as well as nocturnal hypertension. Animal models of simulated OSA suggest that it can.42 Several studies have found that OSA is very common in adult populations with hypertension (>30%).43 This association does not prove causality because patients with hypertension and OSA share common, potentially causative, factors such as obesity. Carlson and coworkers44 found that age, obesity, and sleep apnea were independent and additive risk factors for the presence of hypertension. The Sleep Heart Health Study, a large population-based investigation, found an increased risk of having self-reported hypertension when OSA was present (Table 17–2).45 TABLE 17–2 Cardiovascular Disease and Obstructive Sleep Apnea CAD = coronary artery disease; CHF = congestive heart failure; HTN = hypertension. Values are risk (95% confidence interval). *Data from Shahar E, Whitney CW, Redline S, et al: Sleep-disordered breathing and cardiovascular disease. Am J Respir Crit Care Med 2001;163:19–25. †Data from Nieto FJ, Young TB, Lind BK, et al: Association of sleep disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000;283:1829–1836. A prospective study of the Wisconsin cohort found an increase in AHI predicted the development of hypertension in the following 4 years (increased incidence) after adjusting for confounding factors such as obesity, age, and smoking (Table 17–3).46 In contrast to these findings, a recent analysis of the Sleep Heart Health data found that there was no relationship between the AHI and the risk of incident hypertension (risk of developing hypertension in the next 5 yr) when the risk was adjusted for obesity.47 There was a trend for a relationship when the AHI was greater than 30/hr. One problem with the Sleep Heart Health Study is that the population contained relatively few patients with severe OSA. In summary, patients with OSA are likely at risk of developing hypertension. The risk is most significant with severe OSA and is confounded by the presence of obesity. TABLE 17–3 Risk of Incident Hypertension in Obstructive Sleep Apnea (
Consequences of Obstructive Sleep Apnea and the Benefits of Treatment
Pathophysiology
Mortality
AUTHOR
SELECTION
PARTICIPANTS
FINDINGS
Punjabi, 200916Population-based
Sleep Heart Health Cohort
Marin, 200513Clinical cohort—sleep clinic recruited
Snorers, OSA, and matched control population
Yaggi, 200514Clinical cohort
OSA (AHI > 5/hr) was associated with increased risk of death from any cause.
Patients referred to sleep clinic
RDI > 30/hr males had higher mortality but only in males aged < 50 yr.
Excessive Daytime Sleepiness and Neurocognitive Dysfunction
Automobile Accidents and Sleep Apnea
Arterial Hypertension
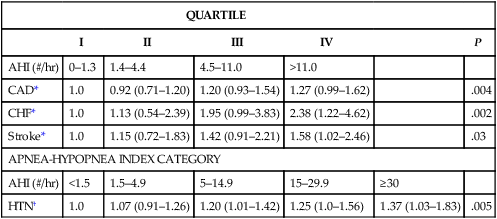
Sleep Heart Health Study—Risk of Self Reported Cardiovascular Disease (Prevalence)
QUARTILE
I
II
III
IV
P
AHI (#/hr)
0–1.3
1.4–4.4
4.5–11.0
>11.0
CAD*
1.0
0.92 (0.71–1.20)
1.20 (0.93–1.54)
1.27 (0.99–1.62)
.004
CHF*
1.0
1.13 (0.54–2.39)
1.95 (0.99–3.83)
2.38 (1.22–4.62)
.002
Stroke*
1.0
1.15 (0.72–1.83)
1.42 (0.91–2.21)
1.58 (1.02–2.46)
.03
APNEA-HYPOPNEA INDEX CATEGORY
AHI (#/hr)
<1.5
1.5–4.9
5–14.9
15–29.9
≥30
HTN†
1.0
1.07 (0.91–1.26)
1.20 (1.01–1.42)
1.25 (1.0–1.56)
1.37 (1.03–1.83)
.005
Wisconsin Cohort—Population-Based)*
QUARTILE
I
II
III
IV
P
AHI (#/hr)
0
0.1–4.9
5.0–14.9
>15
Relative risk (CI)
1.0
1.42
1.13–1.78
2.03
1.29–3.17
2.89
1.46–5.64
.002 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Consequences of Obstructive Sleep Apnea and the Benefits of Treatment





