Fig. 13.1
ACTH adenoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. The pituitary gland is enlarged, with heterogeneous enhancement and nodular erosion of the left sellar floor. The pituitary stalk is mildly deviated to the right
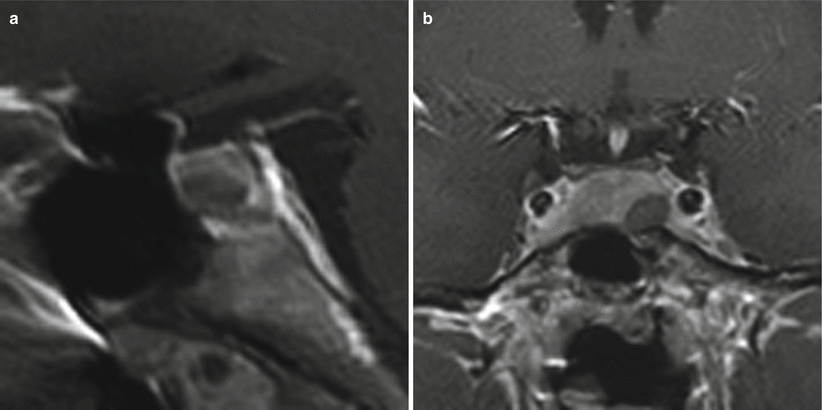
Fig. 13.2
Adrenocorticotropic hormone (ACTH)–secreting adenoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. A focal hypoenhancing mass presents in the left inferior aspect of the pituitary gland. The mass appears separate from the left cavernous internal carotid artery
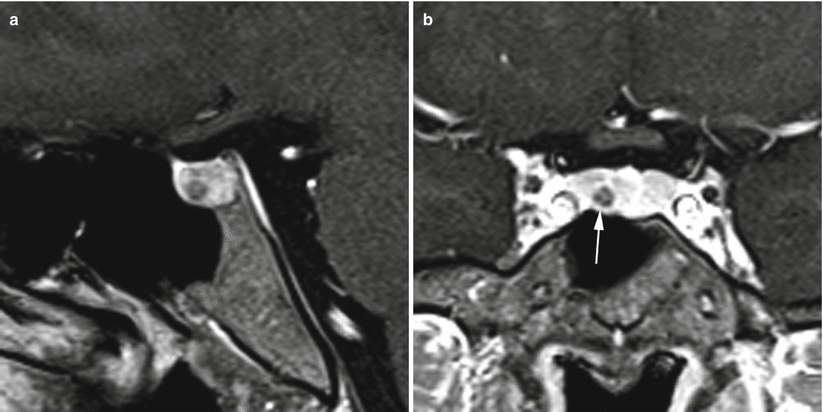
Fig. 13.3
ACTH adenoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. There is a small, hypoenhancing mass (white arrow) in the inferior aspect of the pituitary gland right to midline
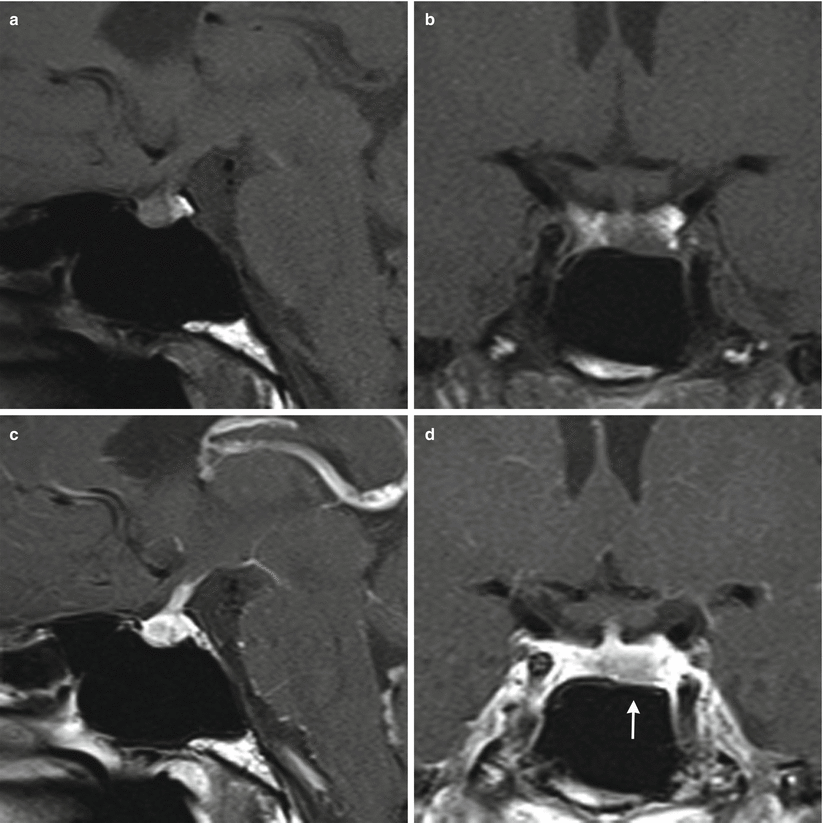
Fig. 13.4
ACTH adenoma. (a) Sagittal T1-weighted pre-gadolinium image. (b) Coronal T1-weighted pre-gadolinium image. (c) Sagittal T1-weighted post-gadolinium image. (d) Coronal T1-weighted post-gadolinium image. A hypoenhancing region is located in the inferior left aspect of the pituitary gland (white arrow), with subtle erosion of the sellar floor
Fig. 13.5
ACTH adenoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. There is an enhancing mass eroding the sellar floor, with suprasellar extension. The mass abuts the cavernous internal carotid arteries bilaterally
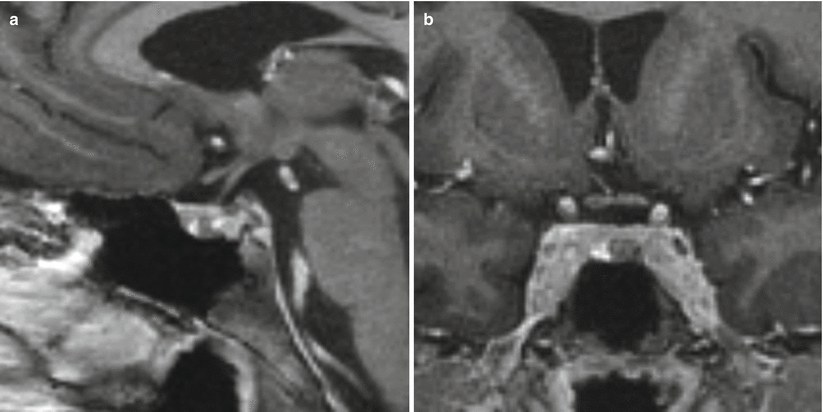
Fig. 13.6
ACTH adenoma. (a) Sagittal T1-weighted post-gadolinium image. (b) Coronal T1-weighted post-gadolinium image. A hypoenhancing mass is seen in the left inferior aspect of the pituitary gland
13.2.2 Inferior Petrosal Sinus Sampling
In some cases, inferior petrosal sinus sampling (IPSS) may be performed to confirm the diagnosis of central ACTH secretion and CD or in the event of suspected CD with a negative MRI.
IPSS offers a sensitivity and specificity of 92–100 % for the diagnosis of CD.
Following administration of CRH, ACTH levels are serially measured from both inferior petrosal sinuses, cavernous sinuses, and the inferior vena cava in order to calculate central–peripheral gradients and lateralization measurements.
13.2.3 Histopathology
Corticotroph (ACTH) adenomas show positive immunoreactivity for ACTH immunostain and periodic acid–Schiff (PAS) [1].
Densely granulated (basophilic) ACTH adenomas (50 %) are strongly PAS and CAM 5.2 positive.
Sparsely granulated (chromophobic) ACTH adenomas are weakly PAS positive and have more frequent cellular pleomorphism.
“Crooke’s hyaline change” refers to a reactive process in adenohypophyseal cells in the setting of chronic hypercortisolemia. The presence of Crooke’s hyaline change without an adenoma has been reported in up to 1.8 % of all sellar lesions, possibly due to intraoperative loss of a microadenoma specimen [1].
“Crooke’s cell adenoma” refers to a corticotroph adenoma displaying larger number of cells with Crooke’s hyaline change (Fig. 13.7).
Multiple ACTH adenomas have been reported in patients with Cushing’s disease [20].
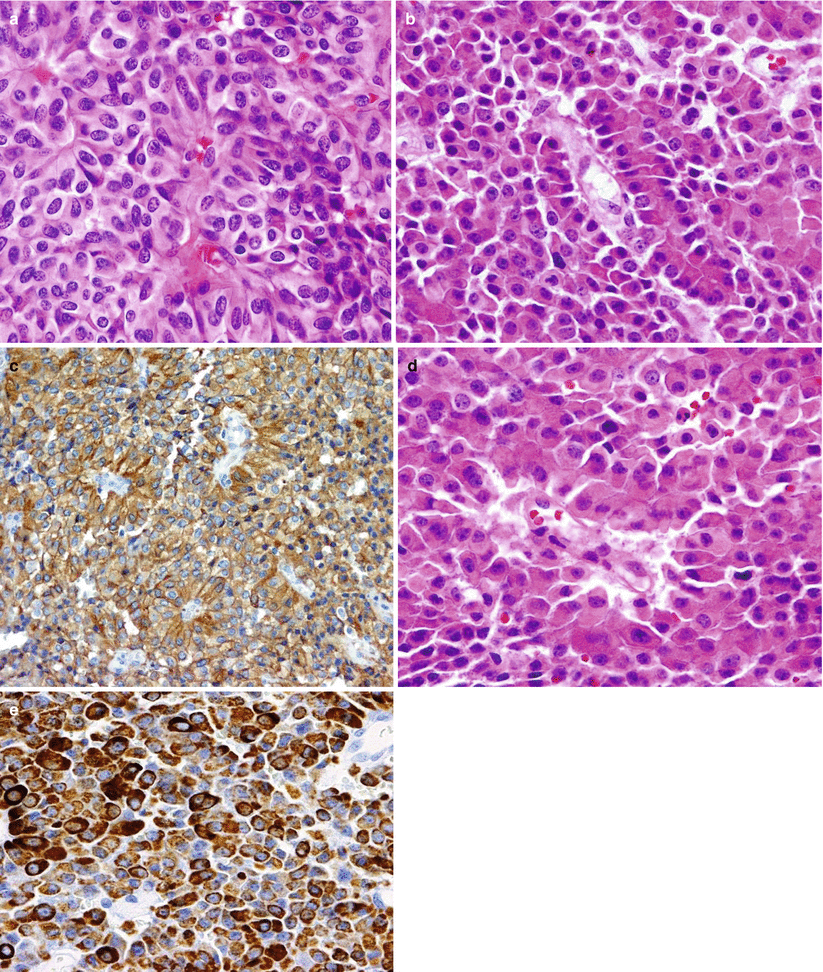
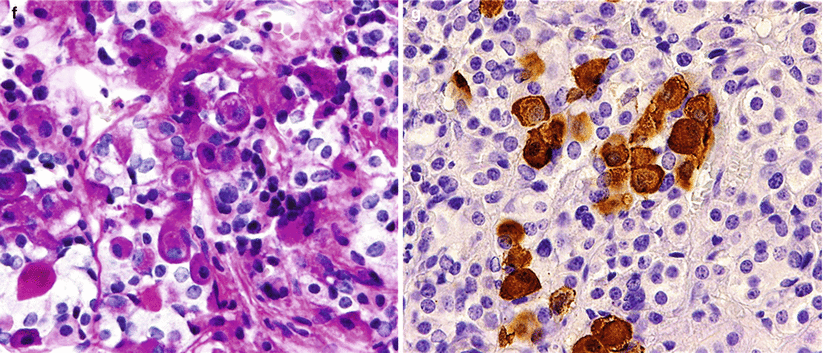
Fig. 13.7
ACTH adenoma. (a) Corticotroph (ACTH) cell adenomas are composed of large cells with angular, amphophilic to basophilic cytoplasm and large pleomorphic nuclei. (b) A tile-like arrangement of cells is sometimes seen. (c) Intense ACTH immunoreactivity in a papillary corticotroph adenoma. (d) A special variant of corticot roph adenomas has an increased accumulation of hyaline bundles in the cytoplasm, characterizing Crooke’s changes. (e) These so-called Crooke’s adenomas show extensive immunoreactivity of the cells, with CAM 5.2 cytokeratin highlighting the Crooke’s changes. (f–g) Crooke’s changes are commonly seen in the normal corticotroph cells of the adjacent pituitary as seen by H&E stain (f) or by the negative reaction of the cytoplasmic hyaline material on ACTH immunostain (g)
13.3 Clinical Management
Surgery remains the primary option for patients with CD. Following exhaustive surgical management, multimodal therapy may be required for patients with persistent hypercortisolemia who do not obtain hormonal remission. Adjunctive treatments following surgical management for CD include radiosurgery and radiotherapy, medical therapy, and bilateral adrenalectomy.
13.3.1 Surgical Management
The primary treatment for CD remains surgical resection.
Endoscopic techniques may provide improved visualization, especially in cases that are MRI negative.
During surgery, a wide exposure of the entire sella and pituitary gland is routinely performed, even in cases of lateralized microadenomas, in order to survey the entire gland.
Extracapsular dissection of pituitary microadenomas should be attempted when a pseudocapsule exists [21].
Following tumor resection, the remainder of the gland is inspected thoroughly for evidence of additional tumors, as multiple ACTH adenomas have been reported in CD patients [20].
During the first several days after surgical resection, cortisol levels are typically evaluated every 6 h until a nadir cortisol level (optimally <2 μg/dL) is reached [22]. Replacement therapy (i.e., hydrocortisone) may be initiated at this time, especially if the patient becomes symptomatic [23].
Parenteral (intravenous) hydrocortisone replacement may be transiently required if oral medications are not tolerated because of nausea and vomiting.
Successful surgical remission in Cushing’s disease is defined as a normal (or preferably subnormal) postoperative 24-h urinary free cortisol level. In Nelson’s syndrome, a normalized ACTH level defines endocrinological remission.
An overnight low-dose DST may be performed on postoperative day 3; a cortisol level less than 3 μg/dL may be used to predict remission in over 90 % of patients [24].
If cortisol levels do not reach subnormal levels following surgery, immediate reoperation and gland reexploration should be considered, especially if histopathology was negative for tumor.
Delayed endocrinological remission, with a mean time to remission of 38 days, has been reported in 5.6 % of patients following surgery and is associated with higher recurrence rates [25].
In patients without early remission, early reoperation (within 60 days), usually for hemihypophysectomy or total hypophysectomy, has resulted in remission in 38–67 % [26, 27].
Rates of remission following transsphenoidal surgery for ACTH-producing tumors have been reported to be 70–90 % for patients with noninvasive microadenomas and approximately 25–50 % for patients with invasive macroadenomas [2, 26, 28, 29].
Recurrence occurs in approximately 17 % of patients achieving initial remission [3].
13.3.2 Medical Management
Medical management of CD remains somewhat limited.
Inhibitors of adrenal steroid synthesis, such as the antifungal agent ketoconazole, have been reported to reduce serum cortisol levels in approximately 70 % of patients, but significant adverse effects have been associated with this medication and other inhibitors of adrenal steroid synthesis. These effects include gastrointestinal distress, gynecomastia, decreased libido, and impotence. In addition, hepatotoxicity has been reported in up to 12 % of patients, mandating frequent liver function laboratory assessments [30].
Cortisol receptor antagonists such as mifepristone (RU-486) are currently being evaluated as potential medical agents for CD. Mifepristone is a glucocorticoid receptor blocker that blunts the effects of cortisol despite raising cortisol levels. Adverse effects include an increased risk of adrenal insufficiency and hypokalemia [31–33].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








