Cranial Nerve VII (The Facial Nerve)
Anatomy of Cranial Nerve VII (Facial Nerve)
Cranial nerve VII (the facial nerve) (Fig. 10.1) is a mixed nerve with both motor and sensory components. Fibers from the motor division supply the facial mimetic musculature, the stapedius, the stylohyoid, and the posterior belly of the digastric [4,33]. In addition, sensation of taste from the anterior two-thirds of the tongue and parasympathetic fibers is carried in a minor root called the nervus intermedius (of Wrisberg).
Motor Division
Fibers of the motor division arise from the motor facial nucleus, which lies in the reticular formation of the caudal pontine tegmentum, dorsal to the superior olive, medial to the nucleus of the spinal tract of cranial nerve V (trigeminal nerve), and anterolateral to the nucleus of cranial nerve VI (abducens nerve). The facial nucleus is made up of four separate longitudinally oriented cell groups (subnuclei) that supply specific muscle groups [39]: (a) the dorsomedial group to the auricular and occipital muscles, (b) the intermediate group to the frontalis and corrugator muscles, (c) the ventromedial group to the platysma, and (d) the lateral group to the buccinator and buccolabial muscles. The orbicularis oculi motor neurons are localized to a cap or cluster in the dorsolateral margin of the dorsal part of the facial nucleus. A different somatotopic organization, however, has been proposed, with facial motor neurons innervating the lower facial muscles primarily found in the lateral part of the nucleus, those supplying the upper facial muscles found in the dorsal part of the nucleus, and those innervating the platysma and posterior auricular muscles found in the medial part of the nucleus [107]. The intrapontine roots arise dorsally from the motor nucleus and run rostrally and dorsally (the ascending intrapontine root) to the level of the nucleus of cranial nerve VI. The root then sweeps over the dorsal surface of the abducens nucleus (as the genu of the facial nerve) and then passes ventrolaterally and caudally through the pons to emerge on the lateral aspect of the brainstem.
The supranuclear control of facial movements occurs through corticobulbar fibers originating from the lower third of the precentral gyrus. These fibers course through the corona radiata, the genu of the internal capsule, and the medial portion of the cerebral peduncle to reach the pons. In the pons most fibers decussate, ending in the facial motor nucleus of the contralateral side. The ventral part of the facial nucleus, which innervates the lower two-thirds of the face, has a predominantly crossed supranuclear control. With supranuclear lesions, the dorsal portion, which supplies the upper third of the face, has been thought to be spared because it has bilateral supranuclear control. Others have proposed that descending corticofacial fibers innervate the lower facial motor nuclear region bilaterally, although with contralateral predominance, and that the upper facial motor nuclear region receives scant direct cortical innervation from either side of the brain [107].
This schema of supranuclear facial muscle control holds true for voluntary facial movements. Emotional involuntary movements and voluntary facial movements may be clinically dissociated, and therefore, a separate supranuclear pathway probably exists for the control of involuntary movements. Spontaneous smiling, but not the voluntary drawing of the corners of the mouth to say “cheese,” is restricted in case of lesions of the contralateral striatum, globus pallidus, hypothalamus, and thalamus. Fibers mediating emotional facial movements do not descend in the internal capsule in their course to the facial motor nuclei. The right cerebral hemisphere is also involved in controlling the supranuclear emotional facial movement and is “dominant” for the expression of facial emotion [30].
Nervus Intermedius (of Wrisberg)
The nervus intermedius is the sensory and parasympathetic division of the facial nerve. It carries preganglionic parasympathetic fibers to the submaxillary ganglion (postganglionic fibers go to the submandibular and sublingual glands) and the pterygopalatine or sphenopalatine ganglion (postganglionic fibers go to the lacrimal, palatal, and nasal glands). The nervus intermedius also receives sensory fibers from the geniculate ganglion, which receives fibers that carry the sensation of taste from the anterior two-thirds of the tongue and also receives afferents from the mucosa of the pharynx, nose, and palate and from the skin of the external auditory meatus, lateral pinna, and mastoid.
The parasympathetic fibers arise in the superior salivatory nucleus of the pontine tegmentum; those controlling lacrimation arise from an associated nuclear mass, the lacrimal nucleus. The gustatory afferents end primarily in the nucleus of the tractus solitarius of the medulla, and the exteroceptive afferents end in the nucleus of the spinal tract of cranial nerve V in the medulla. Some proprioceptive afferents from the facial musculature also travel in the facial nerve and have their perikarya in the mesencephalic trigeminal nucleus.
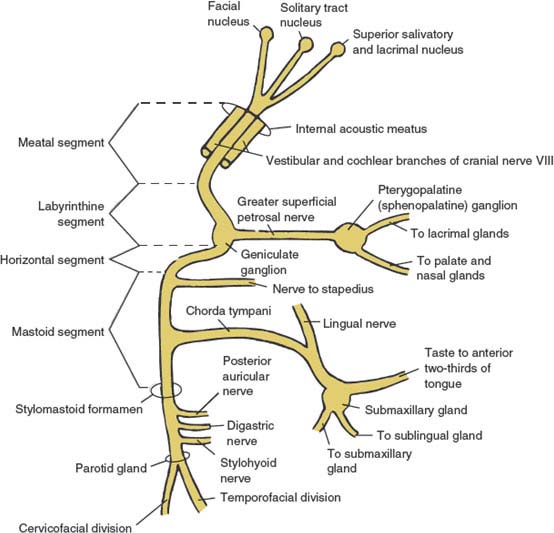
FIG. 10.1. Schematic diagram of cranial nerve VII (facial nerve).
The sensory fibers of the nervus intermedius travel through the substance of the pons lateral to the motor fibers. Together with the motor divisions of cranial nerve VII medially, and cranial nerve VIII (auditory nerve) laterally, the nervus intermedius leaves the pons in the cerebellopontine angle and enters the internal auditory meatus.
Tears are produced by the lacrimal glands (reflex tear secretion), the accessory lacrimal glands of Krause and Wolfring (basal tear secretion), and the goblet cells of the conjunctiva [126]. Preganglionic parasympathetic neurons responsible for lacrimal secretion arise from the lacrimal nucleus of the pons. Their axons travel in the nervus intermedius, which passes through the cistern of the cerebellopontine angle to join the facial nerve; this nerve enters the internal auditory meatus within the petrous pyramid of the temporal bone. Within the petrous bone, the axons destined for the lacrimal gland course through the geniculate ganglion without synapsing and then separate from the facial nerve to emerge from the temporal bone in the floor of the middle fossa as the greater superficial petrosal nerve. The greater superficial petrosal nerve passes under the gasserian ganglion and enters the vidian canal at the anterior end of the foramen lacerum, where it joins the deep petrosal nerve from the carotid sympathetic plexus to form the vidian nerve. This nerve passes to the sphenopalatine ganglion in the pterygopalatine fossa, where the preganglionic lacrimal axons synapse with the postganglionic neurons. The postganglionic axons leave the ganglion and enter the maxillary division of the trigeminal nerve and travel into the inferior orbital fissure with its zygomatic branch. They run in the lateral orbit and reach the lacrimal gland through the anastomosis between the zygomaticotemporal branch of this division and the lacrimal nerve, a branch of the ophthalmic division of the trigeminal nerve [96].
Anatomy of the Peripheral Course of the Facial Nerve
After emerging from the ventrolateral pons, the motor division and the nervus intermedius proceed laterally in the cerebellopontine angle along with cranial nerve VIII. This nerve then enters the internal auditory meatus of the temporal bone together with the auditory nerve and the internal auditory artery and vein. Four portions of the facial nerve can be distinguished within the temporal bone.
THE MEATAL (CANAL) SEGMENT
On entering the meatus, the motor division lies on the superoanterior surface of cranial nerve VIII, with the nervus intermedius between this division and cranial nerve VIII. Within this segment, the facial nerve runs in close association with the vestibular and cochlear divisions of cranial nerve VIII. There are no major branches from this segment of the facial nerve.
THE LABYRINTHINE SEGMENT
At the lateral end of the internal auditory meatus, the motor division and the nervus intermedius enter the facial or fallopian canal in the petrous bone. The labyrinthine segment runs almost at right angles to the petrous pyramid and courses anterolaterally above the labyrinth to reach the geniculate ganglion, which contains the pseudounipolar perikarya of the sensory fibers of the nervus intermedius. The first major branch of the facial nerve, the greater superficial petrosal nerve, arises from the apex of the geniculate ganglion. This nerve is composed of preganglionic parasympathetic efferents that innervate the lacrimal, palatal, and nasal glands through the pterygopalatine (sphenopalatine) ganglion. The greater superficial petrosal nerve also contains cutaneous sensory afferent fibers arising from the skin of the external auditory meatus, lateral pinna, and mastoid.
THE HORIZONTAL (TYMPANIC) SEGMENT
From the geniculate ganglion, the facial nerve runs horizontally backward, below and medial to the horizontal semicircular canal. No major branches of the facial nerve originate from this segment.
THE MASTOID (VERTICAL) SEGMENT
At the posterior aspect of the middle ear (sinus tympani) the facial nerve again changes course and bends inferiorly as the mastoid segment. The nerve to the stapedius muscle originates near the upper end of this segment. The other major branch of this segment is the chorda tympani, which has a variable location of origin. The chorda tympani joins the lingual nerve and contains preganglionic parasympathetic fibers (originating in the superior salivatory nucleus), which innervate the submandibular and sublingual glands through the submaxillary ganglion. The chorda tympani also contains afferent taste fibers from the anterior two-thirds of the tongue destined for the nucleus of the solitary tract.
After giving off the chorda tympani, cranial nerve VII exits the facial canal through the stylomastoid foramen. Near its exit, it gives rise to the posterior auricular nerve (to the occipitalis, posterior auricular, and transverse and oblique auricular muscles), the digastric branch (to the posterior belly of the digastric muscle), and the stylohyoid branch (to the stylohyoid muscles). The facial nerve then pierces the parotid gland where it divides at the pes anserinus into the temporofacial and cervicofacial branches, which further divide into temporofrontal, zygomatic, buccal, marginal mandibular, and cervical branches. These branches supply all the facial mimetic muscles and the platysma muscle.
VASCULAR SUPPLY OF THE FACIAL NERVE
The intracranial portion of the facial nerve is supplied by the anterior inferior cerebellar artery (AICA), and the intrapetrosal portion of the facial nerve is supplied by the superficial branch of the middle meningeal artery and the stylomastoid branch of the posterior auricular artery. The extracranial part of the facial nerve is supplied by the stylomastoid, posterior auricular, superficial temporal, and transverse facial arteries.
Clinical Evaluation of Cranial Nerve VII Function
Facial paralysis can be partial or complete. Diagnosis requires identification of the involved side, underlying etiology, and clinical grading. Many systems for reporting grading of facial function have been proposed. The American Academy of Otolaryngology—Head and Neck Surgery adopted the House-Brackmann six-point subjective grading system (Table 10.1) as its standard [53,97,207].
Motor Function
The motor functions of the innervated facial muscles are assessed by facial inspection and tests of facial mobility. Symmetry of blinking and lip movements with speech is noted. The patient may be asked to raise the eyebrows, wrinkle the brow, close the eyes (orbicularis oculi), show the teeth while repeating a sentence with several labial consonants (orbicularis oris), blow out the cheeks (buccinator), and retract the chin (platysma). Any asymmetry of contraction is noted. The stylohyoid, posterior belly of the digastric, occipitalis, and auricular muscles cannot be adequately tested. However, unilateral loss of ear wiggling, among natural ear wigglers, represents an unusual finding in Bell’s palsy [46]. It must be kept in mind that the facial nerve plays a role in the oropharyngeal phase of deglutition through the buccinator, posterior belly of digastric, perioral, and stylohyoid muscles. Weakness of the stapedius muscle may be detected by the subjective complaint of hyperacusis, especially for low tones that sound louder on the affected side (because the stapedius muscle no longer contracts adequately to tighten the ossicular chain and protect the inner ear from loud noises).
TABLE 10.1 House-Brackmann Classification of Facial Function

Sensory Function
The sensory examination of cranial nerve VII essentially consists of evaluation of taste on the anterior two-thirds of the tongue. Each half of the protruded tongue is tested with the four fundamental tastes (sweet, sour, salty, and bitter) and asymmetries documented.
Reflex Function
The facial nerve provides the efferent supply to several reflexes. The most important of the facial reflexes are the corneal and palpebral reflexes, which are depressed on the side of a lower motor neuron–type facial nerve lesion. Consensual responses are spared. Orbicularis oculi (glabellar), orbicularis oris, and palpebral reflexes may also be depressed with infranuclear lesions.
Parasympathetic Function
Infranuclear facial nerve lesions may result in increased or impaired lacrimation that may be noted subjectively by the patient and can be tested by hanging litmus or filter paper on each lower lid (Schirmer’s test). Excessive salivation may also be noted with infranuclear lesions. Otherwise, facial parasympathetic function is difficult to test objectively at the bedside.
Localization of Lesions Affecting Cranial Nerve VII
Supranuclear Lesions (Central Facial Palsy)
In supranuclear corticobulbar lesions, there is contralateral paresis of the lower portion of the face with relative sparing of upper facial function because the supranuclear control of the upper face has both ipsilateral and contralateral components, whereas the lower face has mainly contralateral supranuclear connections. The muscles around the mouth are especially affected, but there is occasional paresis of the lower or even the upper orbicularis oculi. An alternate explanation for the predominance of lower facial weakness in upper motor neuron facial palsies is that descending corticofacial fibers innervate the lower facial motor nucleus bilaterally, although with contralateral predominance, whereas upper facial motor nuclear regions receive scant direct cortical innervation from either side of the brain [107]. Therefore, upper facial movements are relatively preserved in upper motor neuron palsy because their motor neurons receive little direct cortical input, whereas lower facial muscles are more severely affected because their motor neurons normally depend on significant cortical innervation [107].
Occasionally, there may be a dissociation between voluntary facial movements (volitional facial palsy) and emotional facial movements (emotional or mimetic facial palsy). It is not known which pathways mediate mimetic innervation of facial muscles [81,96].
Volitional facial paresis without emotional paresis (e.g., one side of the orbicularis oris may be paretic when the patient speaks, or he or she may be unable to retract the angle of the mouth on command but does so when spontaneously laughing or crying) is more common than emotional facial paresis and may occur with corticobulbar interruption from lesions of the lower precentral gyrus, internal capsule, cerebral peduncle, or upper pons (above the facial nucleus) [96]. The reverse dissociation, emotional or mimetic facial paresis without volitional facial paresis, occurs with frontal lobe lesions anterior to the precentral gyrus, especially if they affect the right cerebral hemisphere [30]. Unilateral emotional facial paresis has also been described with lesions of the contralateral supplementary motor area, the frontal lobe white matter, the mesial temporal lobe and insula, the striatocapsular territory, the anterolateral thalamus and insula, the thalamus and subthalamus, the posterior thalamus, the posterior thalamus and operculum, and the dorsal midbrain, as well as in postencephalitic parkinsonism [28,79,85,96,193].
Isolated voluntary facial paresis due to a lacunar infarct affecting the contralateral mediodorsal middle base of the pons has been described [196]. This case demonstrates that fibers conveying voluntary orofacial activation descend mediodorsally at the level of the middle pons and that fibers conveying emotional activation may be assumed to converge below this level. The lesion spares corticolingual and corticospinal connections but involves supranuclear corticofacial tract fibers.
Bilateral upper motor neuron lesions result in facial diplegia associated with other manifestations of pseudobulbar palsy (e.g., spastic tongue, dysphagia, uninhibited laughter, and crying).
Nuclear and Fascicular Lesions (Pontine Lesions)
Lesions within the pons may affect either the nucleus of the facial nerve or its intrapontine axons (fascicles). These lesions usually affect neighboring structures, such as the abducens fascicle or nucleus (lateral rectus paralysis), the paramedian pontine reticular formation (PPRF) (paralysis of conjugate gaze to the ipsilateral side), the corticospinal tract (contralateral hemiplegia), and occasionally the spinal tract and nucleus of the trigeminal nerve and the spinothalamic tract (ipsilateral facial and contralateral body sensory disturbances). The association of involvement of these intraparenchymal structures with a facial palsy indicates a pontine lesion.
Nuclear and fascicular lesions of the facial nerve result in a peripheral type of facial nerve palsy. With complete lesions, there is unilateral paralysis of all mimetic facial muscles, with loss of frontal wrinkling and facial asymmetry at rest and with motion. The patient cannot frown or raise the eyebrow, close the eye, retract the angle of the mouth or purse the lips, puff out the cheek, or tighten the chin on the affected side. With mild peripheral affection, only blink asymmetry (incomplete blink on the side of the paresis) may be evident. On attempting to close the eye on the affected side, the eyeball deviates up and slightly outward (Bell’s phenomenon) owing to relaxation of the inferior rectus and contraction of the superior rectus. Bell’s phenomenon is a normal response that becomes visible because of the paralysis of eye closure; this phenomenon may not be present in 8% to 10% of healthy individuals, who instead show no movement or downward eye movements with forced eye closure [75]. The cheek puffs out during respiration, and food tends to accumulate between the teeth and the cheek on the affected side owing to buccinator paralysis. This peripheral type of facial paralysis also results in depressed corneal and palpebral reflexes on the affected side with intact consensual responses and ipsilateral hyperacusis.
MILLARD-GUBLER SYNDROME
Millard-Gubler syndrome is caused by a lesion located in the ventral pons that destroys the fascicles of the facial and abducens nerves and the corticospinal tract. It is characterized by the following signs:
1. Ipsilateral peripheral-type facial paralysis
2. Ipsilateral lateral rectus paralysis (diplopia with failure to abduct the ipsilateral eye)
3. Contralateral hemiplegia
FOVILLE SYNDROME
Foville syndrome is caused by a lesion located in the pontine tegmentum that destroys the fascicle of the facial nerve, the PPRF, and the corticospinal tract. It is characterized by the following signs:
1. Ipsilateral peripheral-type facial paralysis
2. Paralysis of conjugate gaze to the side of the lesion
3. Contralateral hemiplegia
EIGHT-AND-A-HALF SYNDROME
Eight-and-a-half syndrome is caused by a lesion in the dorsal tegmentum of the caudal pons involving the PPRF or abducens nucleus and the medial longitudinal fasciculus (MLF), as well as the nucleus and fasciculus of the facial nerve [65]. It is characterized by the following signs:
1. Internuclear ophthalmoplegia (INO) in addition to horizontal gaze palsy (one-and-a-half syndrome)
2. Ipsilateral lower motor neuron-type facial palsy
ISOLATED PERIPHERAL FACIAL AND ABDUCENS NERVE PALSY
Isolated peripheral facial and abducens nerve palsy is a syndrome caused by a discrete lesion in the caudal tegmental pons involving the facial nerve fascicle (or nucleus) and the abducens nerve fascicle [170]. It is characterized by the following signs:
1. Peripheral-type facial palsy
2. Ipsilateral abduction weakness
3. No other neurologic abnormalities
Posterior Fossa Lesions (Cerebellopontine Angle Lesions)
In the posterior fossa, the motor division of the facial nerve is in close proximity to the nervus intermedius of Wrisberg and the eighth cranial nerve. Lesions in this location (e.g., acoustic neuroma, meningioma) result in the following:
1. Ipsilateral peripheral-type facial nerve paralysis (including loss of taste over the ipsilateral anterior two-thirds of the tongue) without hyperacusis (caused by associated eighth cranial nerve affection)
2. Ipsilateral tinnitus, deafness, and vertigo
Cerebellopontine angle lesions frequently extend to involve other neighboring structures, including the pons (nystagmus or ipsilateral gaze palsy), the cerebellar peduncles and cerebellum (ipsilateral ataxia), the trigeminal nerve (ipsilateral facial pain and sensory changes), and the abducens nerve (ipsilateral lateral rectus paralysis). Affection of cranial nerves IX through XII may rarely occur.
Lesions Affecting the Meatal (Canal) Segment of the Facial Nerve in the Temporal Bone
In the temporal bone, the facial nerve is closely associated with the auditory nerve; therefore, lesions cause clinical findings similar to those seen with the cerebellopontine angle syndrome: unilateral facial motor paralysis, impairment of taste over the ipsilateral anterior two-thirds of the tongue, impaired lacrimation, and deafness (rather than hyperacusis). This syndrome is most often caused by temporal bone fracture and primary or secondary tumors.
Lesions Affecting the Facial Nerve Within the Facial Canal Distal to the Meatal Segment but Proximal to the Departure of the Nerve to the Stapedius Muscle
Lesions within the facial canal distal to the meatal segment but proximal to the departure of the nerve to the stapedius muscle involve the motor division of the facial nerve and the nervus intermedius. There is no deafness or involvement of other cranial nerves. The lesions result in ipsilateral facial motor paralysis, loss of taste over the anterior two-thirds of the tongue, and hyperacusis. If the lesion is proximal to the greater superficial petrosal nerve, lacrimation is impaired; if it is distal to this branch, lacrimation is normal. When the geniculate ganglion is injured, pain may occur in the region of the eardrum. Involvement of the geniculate ganglion by reactivation of latent varicella zoster virus (VZV) results in facial paralysis, hyperacusis, and loss of taste associated with geniculate neuralgia and herpetic vesicles on the eardrum, external auditory meatus, or palate (Ramsay Hunt syndrome). Hyperemia of the concha or helix occurs in some patients. A variable degree of vestibulocochlear dysfunction occurs in approximately 20% of patients. Sometimes, facial paralysis develops without herpetic eruption, a condition known as zoster sine herpete.
Lesions Affecting the Facial Nerve Within the Facial Canal Between the Departure of the Nerve to the Stapedius and the Departure of the Chorda Tympani
Lesions within the facial canal between the departure of the nerve to the stapedius and the departure of the chorda tympani cause facial motor paralysis with loss of taste on the anterior two-thirds of the tongue. Because the lesion is distal to the nerve to the stapedius, hearing is spared (no hyperacusis).
Lesions Affecting the Facial Nerve in the Facial Canal Distal to the Departure of the Chorda Tympani
Lesions in the facial canal distal to the departure of the chorda tympani (e.g., lesions at the stylomastoid foramen) cause facial motor paralysis without associated hyperacusis or loss of taste.
Lesions Distal to the Stylomastoid Foramen
Lesions distal to the stylomastoid foramen produce isolated facial motor paralysis. Individual motor branches of the facial nerve may be affected, thereby causing paralysis of individual facial muscles. In this location, the fibers of the facial nerve may be involved by inflammation of the retromandibular lymph nodes or by tumors or infections (e.g., sarcoidosis, infectious mononucleosis) of the parotid gland. The facial nerve or its branches are also susceptible to facial trauma (e.g., by obstetric forceps) and other surgical misadventures [129]. Other causes of peripheral facial nerve palsy are listed in Table 10.2 and include Lyme disease, leprosy, hepatitis E, acquired immunodeficiency syndrome (AIDS), and leukemia [23,27,49,91,92,208]. A peripheral facial nerve palsy has rarely been described in patients with inflammatory pseudotumor of the middle ear [121], or with acupuncture treatment for temporomandibular joint dysfunction [172]. A peripheral facial nerve palsy has also been reported with the lateral medullary syndrome of Wallenberg and attributed to the involvement of the facial nucleus or intra-axial facial nerve fascicles resulting from the extension of the lesion in the lower pons.
A familial syndrome of hyperostosis cranialis interna may cause recurrent facial nerve palsies (cranial nerves I, II, and VIII may also be affected) [21]. This autosomal dominant disorder causes cranial neuropathies through hyperostosis and osteosclerosis of the calvaria and base of the skull. Other forms of facial paralysis that can be inherited in an autosomal dominant manner include idiopathic familial facial nerve paralysis, Melkersson-Rosenthal syndrome, Möbius syndrome, and hereditary neuropathies with liability to pressure palsies [50].
Idiopathic peripheral facial palsy (Bell’s palsy) is one of the most common conditions seen in neurologic practice, accounting for approximately 50% of the cases of peripheral facial paralysis [25,81]. Women are further at risk when pregnant [95]. The last trimester of pregnancy is considered to be a time for increased risk for the development of Bell’s palsy. Women who develop Bell’s palsy during pregnancy or puerperium should be closely monitored for preeclampsia or arterial hypertension [184]. Idiopathic bilateral facial paralysis, although unusual, is also more frequent during the last trimester of pregnancy or early puerperium [114]. The incidence of Bell’s palsy is also higher in patients with diabetes as compared with the general population. No relationship has been demonstrated between atmospheric fluctuations and Bell’s palsy [56].
Usually, unilateral, clinical, immunologic, serologic, and histopathologic findings implicate the reactivation of herpes simplex virus (HSV) within the geniculate ganglion as the major cause of Bell’s palsy [3]. Other viruses implicated in the etiology of idiopathic peripheral facial paralysis include VZV, cytomegalovirus (CMV), Epstein-Barr virus (EBV), mumps, and human herpes virus 6. Vesicles behind the ear, within the external meatus, or palate should raise a suspicion of Ramsay Hunt syndrome. An increased risk for Bell’s palsy was also reported with the administration of an intranasal inactivated influenza vaccine in Switzerland; as a result of these observations, the intranasal vaccine was withdrawn from the market [148]. Facial paralysis has been associated with influenza vaccination [47,190], hepatitis B vaccination [5], interferon α and ribavirin [19], and nasal vaccines against the human immunodeficiency virus (HIV) and tuberculosis using a nontoxic mutant of Escherichia coli [124].
Weakness confined to one or two facial muscles on the same side of the face may be due to facial trauma, parotid gland neoplasm, or perineural spread of skin cancer [145].
Inflammation and edema of the facial nerve are implicated as the cause of Bell’s palsy. Histopathologic and clinical evidence suggest that the site of the lesion is within the confines of the fallopian canal, particularly at its medial end. Most patients become aware of their facial palsy after awakening. Patients often complain of acute onset of retroauricular pain, dysgeusia, hyperacusis, and decreased tearing [2,3]. Retroauricular pain usually occurs around the time of onset of facial paralysis but may precede its onset by at least 2 weeks [45]. The facial paralysis is often maximal at onset or may progress for over 24 to 48 hours. Careful otoneurologic examination is usually normal except for variable loss of function of the seventh cranial nerve. Transitory numbness of the face in one or more divisions of the trigeminal nerve is also often reported in approximately 25% of patients [2,3].
A small percentage of patients have associated dysfunction of other cranial nerves. According to Adour et al. [2,3], idiopathic facial paralysis is part of cranial polyneuritis, often involving the trigeminal, glossopharyngeal, cochleovestibular, and contralateral (clinically unaffected) facial nerves. Patients with facial paralysis may also suffer from problems with eating and drinking, and transient disturbance or oropharyngeal swallowing has been demonstrated electrophysiologically in approximately two-thirds of patients [181].
TABLE 10.2 Etiologies of Peripheral Facial Nerve Palsies

Patients with unilateral facial paralysis are at risk of developing corneal ulceration because of lagophthalmos. Lack of function leads to ectropion. Ensuring adequate corneal protection is the immediate ophthalmic priority. Some patients may complain of epiphora, or, conversely, of a dry eye. Bell’s palsy is a self-limiting condition. Most patients have a favorable prognosis. However, some develop disabling sequelae. Patients with reactivation of VZV infection or loss of the stapedial reflex may have a poorer prognosis for full recovery. Rarely, recovery may be followed by transient or long-lasting motor dysfunction such as motor synkinesis, myokymia, blepharospasm-like activity, or hemifacial mass contractions associated with normal facial movements. In some cases, aberrant regeneration may cause involuntary tearing of the eye on the involved side (crocodile tears, Bogorad’s syndrome), or gustatory sweating (Frey’s syndrome) when parasympathetic fibers to the salivary glands reinnervate the sweat glands [197]. Recurrence occurs in approximately 7% of patients with Bell’s palsy [103].
Occult parotid malignancies rarely present with acute-onset unilateral facial paralysis [162]. In highly endemic areas, Lyme disease, an arthropod-borne spirochete (Borrelia burgdorferi) infection known to cause erythema chronicum migrans, headaches, papilledema, cranial neuropathies, meningomyeloneuritis, lymphocytic meningitis, heart block, and arthritis, may be responsible for one-fourth of cases of peripheral facial palsy. Bilateral facial involvement occurs in one-fourth to one-third of cases [24,41].
Recurrent orofacial swelling predominantly affecting the lips, face, and eyelids; unilateral or bilateral facial nerve palsy; cheilitis; and fissured tongue (scrotal tongue or lingua plicata) define Melkersson-Rosenthal syndrome [64,88], which may be associated with a variety of disorders, including hyperhidrosis, acroparesthesia, migraine, retrobulbar optic neuritis, paresis of the medial rectus muscle, Crohn’s disease, and seronegative oligoarthritis [64]. The complete syndrome is present in only 25% of patients [88]. Lingua plicata and facial paralysis is seen in approximately half of the patients. Rarely, facial palsy and lingua plicata, two of the main features of the classic triad of Melkersson-Rosenthal syndrome, have been described in association with Waardenburg syndrome, a condition characterized by sensorineural hearing loss; pigmentary disturbances of the hair and iris; and other developmental defects [63]. Facial paralysis and concurrent facial swelling is also an uncommon but well-described complication of infantile cortical hyperostosis, an inflammatory condition of the skeleton and some of the contiguous fasciae and muscles [42]. Rarely, recurrent idiopathic facial nerve palsy is associated with episodes of ophthalmoplegia and familial aggregation [120]. Bilateral facial paralysis (facial diplegia) is uncommon, occurring <1% as frequently as unilateral paralysis [109]. Bilateral involvement may be due to congenital developmental anomalies or associated with infectious, traumatic, postinfectious, granulomatous, or neoplastic processes (Table 10.3) [82,109,187]. Developmental facial diplegia may be secondary to Möbius syndrome of bifacial paresis with abnormalities of horizontal eye movements, usually as a result of bilateral hypoplasia or aplasia of the sixth and seventh cranial nerve nuclei. Developmental facial diplegia may also be associated with Poland’s anomaly (unilateral pectoralis muscle hypoplasia with ipsilateral breast and upper limb abnormalities) [40]. Bilateral facial paralysis may also occur with collagen vascular diseases, hypovitaminosis A associated with cystic fibrosis [37], osteopetrosis, and may rarely be idiopathic (bilateral Bell’s palsies). Weakness of the inferior facial muscles should be differentiated from emotional facial paresis, characterized by weakness of the lower facial musculature evident during emotionally provoked movements but not during voluntary contractions [41]. Bilateral facial paralysis must be differentiated from other causes of facial weakness or loss of facial movements, as seen in myotonic dystrophy, myasthenia gravis, or Parkinson’s disease.
Facial nerve paralysis occurs less frequently in children than in adults and presents special challenges. Most children with Bell’s palsy recover completely [194]. Partial unilateral lower lip palsy in the neonatal period may be caused by involvement of the depressor labii inferioris muscle, innervated by the marginal mandibular branch of the facial nerve. This partial paralysis may be associated with serious cardiac, skeletal, and genitourinary abnormalities [34]. A reversible form of facial paralysis has been associated with the administration of nasal continuous positive airway pressure [130]. Birth trauma resulting from sacral prominence pressure on the fetal face during labor accounts for most cases of facial nerve paresis in the newborn period [40]. Not every facial palsy is a Bell’s palsy. Misdiagnosis of the etiology of unilateral facial weakness is common. Although Bell’s palsy is still by far the most common cause encountered, secondary causes of facial nerve paralysis must be considered in the differential diagnosis of pediatric patients [186]. Facial palsy can result from congenital developmental anomalies, skull fractures, iatrogenic injuries during surgery of the diseased or congenitally malformed ear, hemotympanum associated with hemophilia A, hypertensive hemorrhage in the facial canal, osteopetrosis, and hypertension. Involvement of the facial nerve is common in the Guillain-Barré syndrome or the Fisher variant of the Guillain-Barré syndrome. Prompt diagnosis of recurrent central nervous system leukemia and lymphoma, cerebellopontine angle tumors, yolk sac tumors, and embryonal rhabdomyosarcoma of the middle ear is needed. Careful examination of the middle ear is recommended in children with facial weakness. Middle ear tumors should be considered in the differential diagnosis of unresolved otitis media, particularly when associated with persistent ipsilateral facial paralysis. Cranial nerve palsies are common in tuberculous meningitis and in a variety of infectious and parainfectious disorders including Lyme disease, chicken pox, herpes zoster oticus (Ramsay Hunt syndrome), coxsackievirus, EBV, HIV, human herpes virus 6 infection [158], Mycoplasma pneumonia, Parvovirus B19 infection, cat scratch disease, acute disseminated encephalomyelitis, complications of acute and chronic otitis media or necrotizing otitis externa, and bacterial or mycobacterial mastoiditis. Facial paralysis has also been reported in children in association with Kawasaki disease and as a result of ischemic vasospasm associated with dental blocks [24,36,48,59,77,134,159,166,171,185].
TABLE 10.3 Etiologies of Bilateral Facial Nerve Palsies










