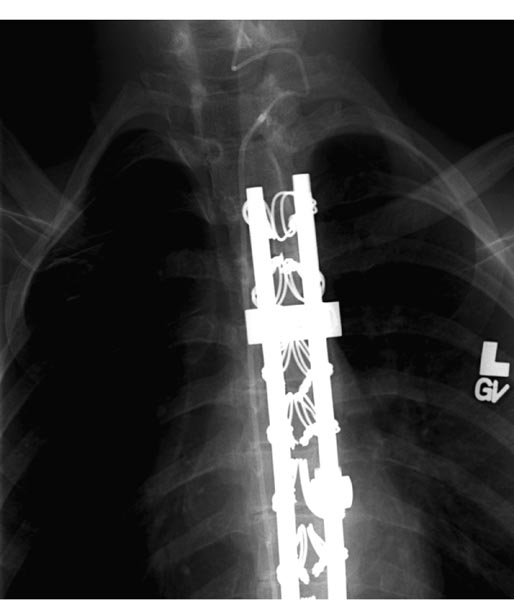
19 Spasticity The pairing of lumbodorsal rhizotomy (LDR) and intrathecal baclofen (ITB) in this book might suggest that the two treatment modalities were competing for the same group of children. In reality, they are complementary; there are almost no children for whom LDR and ITB are equally appropriate. LDR is an excellent treatment for children with spastic diplegia or with spastic quadriparesis but little spasticity in the arms. It can be used effectively into the midteens and occasionally thereafter if patients have adequate underlying strength, and their associated contractures are mild. ITB is indicated in very different circumstances. Although there are few controversies about patient selection for ITB, controversies exist about surgical techniques and about ITB outcomes—such as the relationship between ITB and seizures or the relationship between ITB and the development of scoliosis—and will be discussed here. ITB is infrequently indicated for children with spastic diplegia, but it is indicated for them in three circumstances. 1. It is appropriate for ambulatory children with relatively weak lower extremity muscles but moderate or severe spasticity (3 to 4 on the 5-point Ashworth scale) that is impeding gait and causing progressive contractures, since LDR in these children may result in such hypotonia that they are unable to walk because of their underlying weakness. 2. ITB is appropriate for older individuals (older than 16 years, particularly those in their 20s and 30s) with spastic diplegia whose gait is deteriorating as a result of stiffness or pain because they have a harder time learning better gait patterns after LDR than do young children. ITB is helpful in these patients because their spasticity can be gradually decreased, with baclofen doses increasing as muscles stretch and strengthen with physical therapy. 3. Spastic diplegia in children with hereditary spastic paraparesis (HSP) can be effectively treated with ITB.1In children with HSP, spasticity is typically reduced, although gait is not always better because of their associated proximal leg weakness. In my experience, an LDR in one child with HSP improved spasticity for 6 weeks, at which point it returned to the prerhizotomy level. Spastic quadriparesis is the classic indication for ITB. It is related to cerebral palsy in the majority of cases but may be secondary to severe head injuries. Regardless of etiology, ITB appears to improve spastic quadriparesis.2,3 Although LDR has been shown to decrease spasticity in the upper extremities, that decrease is mild and considerably less than can be obtained with ITB.4 Most children who receive ITB for spastic quadriparesis have mean scores of 3 on the 5-point Ashworth scale in both the upper and lower extremities and have been ineffectively treated by oral medications and botulinum toxin injections. Some clinicians predicted that the use of ITB in spastic hemiparesis would be contraindicated because of concern that the normal side would become hypotonic. In my experience, including with one of the first children to receive a pump in 1988, that is not so: spasticity in the affected side is reduced, and tone in the normal side seems to be unaffected. In patients with spastic hemiparesis, spasticity is usually decreased in the upper extremity, often resulting in improved positioning and functioning of the affected arm and hand. Children with mixed spasticity and dystonia in the lower extremities should not be treated with LDR if there is a significant component of dystonia, as it is unaffected by dorsal rhizotomies (although it may be improved by ventral rhizotomies). Conversely, ITB has been shown to improve both spasticity and dystonia, whether in the lower extremities or in both the upper and lower extremities.5 ITB is usually offered to children who have failed oral medications (e.g., baclofen, tizanidine, and dantrolene), although sometimes children present with such severe generalized spasticity that trials of oral medications seem futile, and a decision is made to proceed directly to ITB. There is no controversy about the fact that the response to oral baclofen correlates poorly with the response to ITB, as so little oral baclofen enters the cerebrospinal fluid (CSF). Before recommending ITB, it is essential to define the treatment goals and to have the parents concur with those goals. Common goals include (1) increasing comfort, (2) increasing ease of care, (3) improving function, and (4) decreasing the development of musculoskeletal contractures. It has been said that “illusion is the parent of disillusion,” and if treatment expectations are inappropriate, disappointment is almost invariable. The reliability of the caregiver to return at appropriate intervals for pump refills should be considered before recommending a pump. We occasionally decide not to put a pump into a child who would probably benefit from it because the parents or caregivers seem to be unreliable. In such cases, we sometimes order a physical therapy regimen twice a week for 6 to 8 weeks, partly to improve the child’s condition prior to surgery, but more so to evaluate the consistency with which the child is brought to therapy. There is considerable controversy about the necessity of screening trials before a pump is inserted. When the use of ITB began some 20 years ago, bolus injections were always given, primarily to determine if intrathecal baclofen significantly decreased lower extremity spasticity. Since then, thousands of screening injections have been given, and we have learned that spasticity virtually always responds, whether of spinal or cerebral origin. Children who are thought not to respond usually have dystonia or athetosis rather than spasticity, although occasionally a dose higher than the usual 50 g is needed to cause a 1-point decrease in mean Ashworth score in the lower extremity. Bolus screening trials are currently advocated in several circumstances: (1) centers that are just starting ITB therapy that have minimal experience with the changes caused by ITB, (2) individual cases where parents insist on demonstrating that a bolus dose decreases tone before submitting their child to a pump, and (3) cases in which a screening dose is mandated by insurance companies. I used screening doses in the first 200 or so spasticity cases but have not used them in patients with spasticity in probably 10 years. If a screening bolus—usually 50 g—is given, the sole purpose of doing the trial is to confirm that spasticity in the lower extremities is decreased. The purpose is not to determine if function, particularly ambulation, is improved. Some investigators have inserted a lumbar catheter and used a continuous baclofen infusion in an attempt to determine if ambulation is improved. That technique provides more information about how ambulation might be affected, but it cannot determine the effects that will be observed during chronic infusion and dose titration over several months. Given the size of the pumps that are presently available, there are no longer any size or age limitations on pump insertion. The Medtronic SynchroMed 20 mL pump (Medtronic Inc., Minneapolis, Minnesota) is small enough to insert into an infant, although the umbilicus may need to be mobilized to obtain adequate space medially. Realistically, however, pumps are often not needed to treat spasticity in children younger than 4 years. ITB is indicated whenever it is clear that nonsurgical management is inadequate, ideally before the development of significant contractures. There is some controversy about the timing of pump insertion in children with posttraumatic spasticity because of a previous recommendation that ITB be delayed for 1 year after the head injury. That recommendation is inappropriate, however. For children with severe posttraumatic spasticity, by 1 year (in fact, often by 6 weeks after injury) spasticity usually has resulted in contractures at several sites, and the children require multilevel orthopedic surgery. In my opinion and the opinion of others, spasticity that develops rapidly after traumatic brain injury should be treated with dantrolene, botulinum toxin injections, and ankle casting, but if definite improvement is not seen within 1 month, ITB should be used.6 A child may improve over the first year after TBI and not require ITB indefinitely. If that is the case, the child at 1 year will have fewer contractures than would have developed otherwise, and the pump can be turned down or removed. Techniques for pump and catheter insertion were reviewed by Albright et al.7 Pumps can be inserted either above or below the abdominal fascia. In young and thin patients, the subfascial position is preferable because it is associated with a considerably better cosmetic appearance and with less tension on the healing skin edges than if the pump is inserted above the fascia. Pumps with a 40 mL reservoir can usually be implanted in children weighing 40 lb or more; smaller children usually receive pumps with a 20 mL reservoir. Pumps with suture eyelets allow the pump to be secured to muscle or fascia more easily than the Dacron pouches that were previously used. Removal of Dacron pouches is difficult and results in the development of a substantial seroma at that site for days afterward. There is controversy over the site where the intrathecal catheter tip should be positioned. For patients with spastic diplegia, there is uniform agreement that the tip should be positioned as it was in the earliest reports of ITB for spastic paraplegia of spinal cord origin: at the T10–T12 region, near the lumbar enlargement of the spinal cord, the site where baclofen will work in these patients. For patients with spastic quadriparesis, catheter tips are currently positioned in either the midthoracic region or the cervicothoracic junction. Grabb et al reported better reduction of spasticity in the upper extremities if the catheter was positioned in the midthoracic region rather than in the lower thoracic region, thinking that the higher catheter tip would result in a higher baclofen concentration in the cervicothoracic CSF.8 I position the tip at C5–T2 for patients with spastic quadriparesis and have observed better upper extremity effects (e.g., decreased tone and increased range of motion) with no increase in side effects. Occasionally, a patient with a cervical catheter will demonstrate less effect in the lower extremities than desired. If the child is fused or is expected to need a spine fusion, it is easier to tunnel the catheter to the cervical region and insert it into the thecal sac via a small laminectomy than to try to insert it by drilling through the lumbar fusion (Fig. 19.1). When replacing an intrathecal catheter, there is debate about the management of the initial catheter. Surgical options include removing the catheter or tying it off and leaving it in place. In my experience, removal is often followed by CSF tracking along the catheter and accumulating around the new pump, sometimes with leakage through the incision. Because of that, it is probably safer to tie off the catheter and leave it in place. After pump insertion, patients are typically kept at flat bed rest for 48 hours in an attempt to prevent CSF leaks around the intrathecal catheter. I know of no data showing that bed rest decreases that risk, but it seems intuitively to make sense. Some neurosurgeons, however, are implanting pumps in adults on an outpatient surgery basis, a policy that seems less appropriate for pediatric patients. Pumps are turned on on the day of surgery and programmed to infuse a priming bolus to fill the catheter, then to infuse baclofen, usually at a constant rate of 100 μg/day when treating spasticity. Doses are increased daily as needed. Because the half-life of intrathecal baclofen is ~4 to 5 hours, and 5 half-lives are needed to reach steady state, doses are increased only once a day. Increases of 10 to 20% per day are recommended, but larger doses may be indicated, depending on patient size, severity of spasticity, and response to the initial infusion. Baclofen is infused in a continuous mode in nearly all patients with spasticity, although additional boluses can be programmed to treat spasticity that is more severe at certain times of the day. Complex dosing can be used if there is inadequate response to higher doses (e.g., 600 μg/day), but there are no data to document better results than seen with continuous infusions. Fig. 19.1 Anteroposterior radiograph demonstrating a catheter tunneled subcutaneously cephalad and inserted into the cervical thecal sac via a small laminectomy. There is controversy about the use of compounded baclofen solutions for ITB. Baclofen is supplied commercially in either 500 μg/mL or 2000 μg/mL solutions. Some pharmacists have compounded baclofen in higher concentrations (e.g., 4000 μg/mL) in an attempt to decrease the frequency of pump refills or to improve patient response. The solubility of baclofen at those concentrations is unreliable; precipitation may occur (within the pump), and high concentrations invalidate the pump warranty. Most patients who require high doses for spasticity (e.g., > 800 μg/day) either have a suboptimally functioning system or have associated dystonia that requires higher ITB doses. Two studies have provided class I evidence of the effect of ITB on spasticity in bolus screening trials.9,10 Numerous other studies, mostly providing Class II to IV evidence, have documented improved spasticity in the lower and upper extremities after chronic ITB administration.3 In 2003, we published the results of a prospective, open-label follow-up study of long-term ITB infusion in 68 patients, 54 with cerebral palsy.11 Their mean age at pump implantation was 12.6 years, and the mean follow-up was 70 months (range 14.6–133.4 months). Spasticity was significantly decreased in both the upper and lower extremities (p<.005) and remained decreased for up to 10 years. ITB has been associated with improved function. Krach et al evaluated Gross Motor Function Measure scores in 31 patients, ages 4 to 29 years, at baseline and 12 months after ITB therapy and found improved scores in all patients in all dimensions except running and walking.12 Awaad et al performed the Pediatric Evaluation of Disability Index (PEDI) in 39 patients receiving ITB therapy, in a prospective, open-label 18-month study.13 They observed improved self-case, improved social function, and less reliance on caregivers in 29 of the 39 subjects. The effects of ITB on gait were reported by Gerszten et al, who graded ambulation in four functional levels (community, household, nonfunctional, and nonambulatory) in 24 patients who were partially ambulatory pre-ITB.14 Ambulation improved by one level in 9, was unchanged in 12, and worsened in 3. The relationship between ITB and seizures is controversial. Although increased seizure frequency has been reported after the administration of oral baclofen, there are few reports that ITB increases seizure frequency. In perhaps the largest series addressing that issue, Buonaguro et al evaluated the effects of ITB on seizures in 150 children.15 Before ITB, 40% of the children had seizures; after ITB, their seizures decreased in 13% and increased in 2%. Of the 60% of children with no seizures before ITB, only one child developed seizures after ITB. The effects of ITB on musculoskeletal deformities are less clear. In 1996, we reported that ITB appeared to reduce the need for subsequent orthopedic surgery in 40 subjects who were evaluated by an orthopedist before and after pump insertion, with a mean follow-up period of 53 months.16 The effects of ITB on hip migration were evaluated by Krach et al in 33 patients whose ages ranged from 4 to 31 years. Postoperatively, the hip migration index was unchanged in 90% in the year after ITB.17 The question about the relationship between ITB and scoliosis is controversial. Sansone et al observed rapid progression of scoliosis in four children after ITB, but in a substantially larger series of 80 patients with gross motor function classification system (GMFCS) level IV or V whose mean age was 11 years, no patient had rapid progression of scoliosis in the year after ITB therapy.18,19 LDR and ITB are excellent treatments for distinctly different clinical indications—in general, LDR for spastic diplegia and ITB for spastic quadriparesis. Relatively good data indicate that ITB decreases spasticity significantly in the upper and lower extremities. Moderately reliable data indicate that ITB improves function, quality of life, and caregiver burden. Controversy about the effect of ITB on scoliosis is related to the paucity of good data about the issue. The treatment of childhood spasticity remains a difficult problem for both health care providers and patients.20 Cerebral palsy is the most common cause of spasticity and physical disability in children. It defines a range of nonprogressive syndromes of posture and motor impairment that results from an insult to the developing central nervous system (CNS) either in utero or within the first 2 years of life.21 The prevalence of cerebral palsy is not known, but it is estimated at ~2 per 1000 children, and its incidence may be increasing due to advances in neonatal intensive care units and improved survival of low birth weight infants.22 Common features of cerebral palsy include movement disorders, muscle weakness, ataxia, rigidity, and spasticity. Spasticity is defined as a velocity-dependent increased resistance to passive muscle stretch, or alternatively, as inappropriate involuntary muscle activity associated with upper motor neuron paralysis.23,24 It is a hallmark of cerebral palsy, but it can occur with other genetic and metabolic diseases that cause dysfunction or damage to the developing CNS. Spasticity is demonstrated in children as increased muscle tone, hyperactive deep tendon reflexes, persistent primitive reflexes, and difficulty with normal motor skills. Spasticity inhibits effective use of motor control and strength and can lead to progressive musculoskeletal complications, such as joint and muscular contractures, bony deformation, and joint subluxation or dislocation.25 Proper treatment for spasticity can halt progression of contractures or deformity and often can return some function to affected limbs. Both prospective and retrospective studies of children treated for spasticity have demonstrated improved ease of caregiving, decreased pain, and increased quality of life.26 Spasticity in children can result from any disease process that affects the upper motor neuron within the CNS. Injury to the upper motor neuron will decrease cortical input to the descending reticulospinal and corticospinal tracts, which causes weakness, loss of motor control, and reduction in the number of voluntarily active motor units. The reduction of these descending tracts removes the normal inhibition of the reflex arcs within the gray matter of the spinal cord, leading to a hyperactive reflex arc and spasticity. In addition to cerebral palsy, spasticity may result from any injury to the brain or spinal cord, including trauma, infection, immunological disorders, and neurodegenerative disease. The diagnosis of spasticity requires a complete history and physical examination, with ancillary testing as needed. The history should inquire about possible gestational and perinatal events, as well as motor and cognitive development. The physical exam should focus on motor power, muscle tone, active and passive range of motion of joints, sensation, deep tendon reflexes, station (pelvis and leg alignment while standing), presence of limb deformity, spinal alignment, and extent of movement disorders. Ancillary testing usually includes imaging studies, particularly magnetic resonance imaging to evaluate for evidence of hemorrhage, hydrocephalus, or structural abnormalities of the CNS. Spasticity is most commonly quantified by the Ashworth spasticity scale (Table 19.1)27,28
Intrathecal Baclofen
Indications for Intrathecal Baclofen in Spasticity
Spastic Diplegia
Spastic Quadriparesis
Spastic Hemiparesis
Mixed Spasticity and Dystonia
Patient Selection
Screening Trials
Timing of Pump Insertion
Surgical Techniques
Patient Management
Outcomes
Conclusion
Selective Dorsal Rhizotomy
Management of Spasticity
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree