Chapter 24 Craniopharyngiomas
Craniopharyngiomas are tumors of neuroepithelial origin that arise from squamous cell rests found along the path of the primitive craniopharyngeal duct. Their incidence ranges between 0.5 and 2.5 per 100,000 person years and does not vary by sex or race. Craniopharyngiomas account for 1.2% to 4.6% of all intracranial tumors (Central Brain Tumor Registry of the United States). They exhibit a bimodal distribution, first peaking during childhood (5–14 years) and later peaking in adults ranging from 50 to 74 years; they comprise 5% to 10% of pediatric brain tumors and 1% to 4% of adult brain tumors.1–4 Craniopharyngiomas have a growth pattern that is often in close proximity to the pituitary infundibulum, and can occur within the sella, suprasellar space, or third ventricle, frequently spanning these spaces. These tumors tend to involve a number of neural structures, including the optic nerves, internal carotid arteries (ICAs), and pituitary gland, causing a variety of symptoms. Common clinical presentations include visual dysfunction with symptoms of chiasmatic as well as postchiasmatic compression, hypothalamic dysfunction with behavioral changes ranging from alterations in eating patterns, to apathy, and even obtundation and pituitary dysfunction, often manifesting as hypopituitarism.
Classification
Several authors who attempted to radiographically classify craniopharyngiomas include Rougerie, Pertuiset, Konovalov, Steno, Hoffman, Samii, and Kassam.5–11 Although none have been universally adopted, these classifications all share the principle of subdividing lesions along the length of extension in the primary vertical axis; the resulting relationship is with the optic chiasm and the third ventricular floor immediately posterior to it.
Anatomy
Another important limiting factor during surgical resection is the close apposition of craniopharyngiomas to the pituitary infundibulum. This opposition has led some to argue that gross total resection cannot be achieved without the section and removal of the involved part of the infundibulum. Although this point remains contested, the morbidity of sacrificing the infundibulum is significant, particularly in pediatric patients.12–19 Diabetes insipidus and hypopituitarism after injury to the infundibulum can impact both the physical and mental growth of patients and limit their daily functional capacity.
Treatment Decision Making
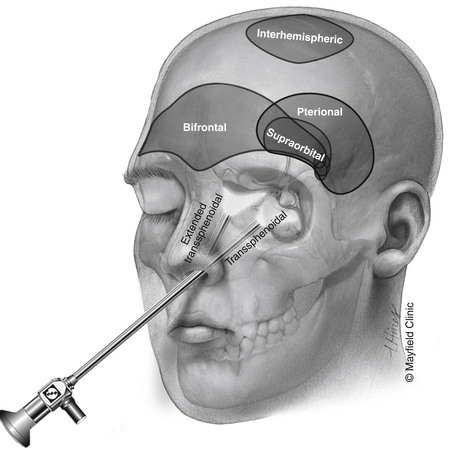
Although most patients undergo both surgical and radiation treatments, a number of questions need to be addressed on an individual level. Choice of surgical approach depends primarily on the extent of the lesion along the vertical axis (Fig. 24-1). Lesions that are purely intrasellar are preferably approached through a trans-sphenoidal approach. Microscopic or endoscopic corridors have been well described and mimic the approach to pituitary adenomas. Because intrasellar lesions are often cystic, obliteration of the tumor cavity with fat may not be indicated unless a cerebrospinal fluid (CSF) leak is manifested intraoperatively, effectively allowing for a prolonged outlet that can delay or prevent future reaccumulation of fluid within the cavity. The trans-sphenoidal route can also be used successfully in the presence of significant suprasellar extension in mostly cystic lesions. The recent development of expanded endoscopic trans-sphenoidal approaches to the sella make the resection of the cyst wall possible when vascular adherence is not a significant issue.
Transcranial routes to the suprasellar space range from a supraorbital corridor to the pterional approach (with or without an orbital or orbitozygomatic osteotomy) to the bifrontal craniotomy. Although each one of these approaches provides a similar exposure to lesions in the suprasellar space, they differ in several important ways. The supraorbital craniotomy, which minimizes soft-tissue morbidity and creates a shorter overall incision, is limited by the size of the frontal sinus. Violation of the frontal sinus through the supraorbital approach can cause a CSF leak that is challenging to fix or a rotation periosteal flap is difficult. Additional limitations of the supraorbital craniotomy include the limited vertical exposure and difficulty with effective brain retraction.
Surgical Techniques
Trans-Sphenoidal/Expanded Trans-Sphenoidal Approach
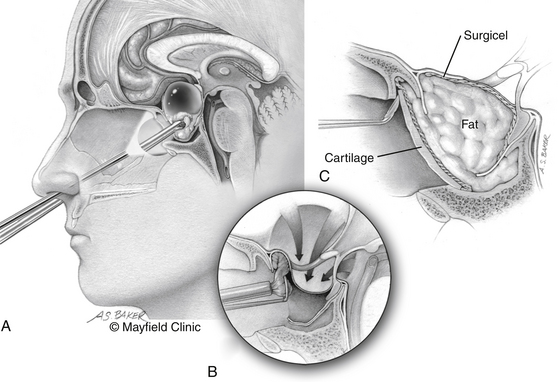
The patient is positioned supine; the head in the “sniffing” position allows for elevation above the level of the heart and drainage of bloody material inferiorly away from the surgical field. The traditional trans-sphenoidal approach through a microscopic or endoscopic approach can be used. Intrasellar tumors and mostly cystic craniopharyngiomas even with a significant suprasellar extension are excellent candidates for this approach (Fig. 24-2).
The expanded trans-sphenoidal approach requires significant bony removal superiorly past the tuberculum. Importantly, at the beginning of an expanded trans-sphenoidal approach, consideration should be given to raising a nasoseptal mucosal flap, which will be used during closure.20 Visualization of the superior aspect of the lesion and its relationship to the undersurface of the optic chiasm is feasible with debulking of the tumor. The perforating vessels and the plane with the optic apparatus need to be sharply dissected under direct vision. However, present endoscopic instrumentation creates an obstacle to effective dissection in many patients.
Although a watertight closure of expanded trans-sphenoidal approaches remains elusive, significant steps have been made during the past several years. Simple fat obliteration proves to be inadequate reconstruction. Multilayer reconstructions and the use of vascularized pedicled mucosal flaps have been promising as an effective reconstruction that can prevent leaks. Use of specialized clips that attempt direct dural reapproximation is an obvious improvement, yet technically very challenging. Deployment of inflatable balloons within the sphenoid sinus can augment the reconstruction. Lumbar subarachnoid drains for temporary CSF diversion offer mechanical advantages but must be balanced by their risk of potential complications, compression of neurovascular structures for the former, and development of pneumocephalus for the latter.
Bifrontal Craniotomy
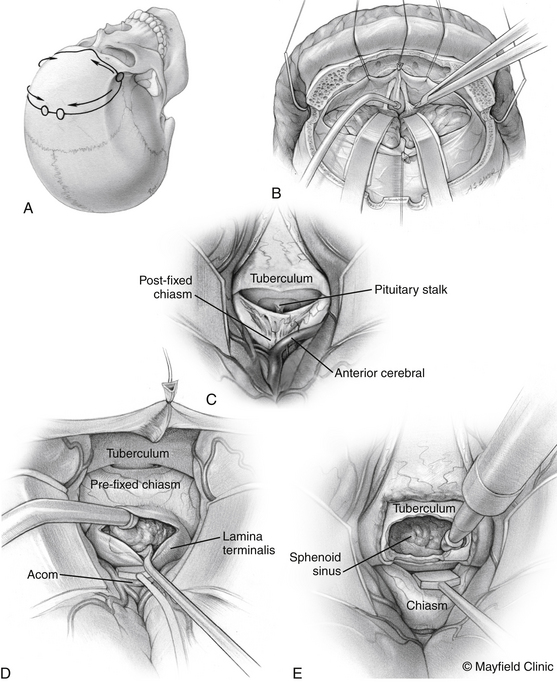
The patient is positioned supine with the head slightly extended in three-point pin fixation in the Mayfield clamp. Frameless stereotactic guidance is a useful adjunct for accurate localization of the lesion. A bifrontal incision is marked behind the hairline. Ensuring that the lateral limits are close to the zygomas bilaterally then limits the pressure on the skin flap and avoids flap ischemia. Burr holes are placed on either side of the superior sagittal sinus, approximately 5 cm above the nasion and laterally superior to the keyhole, just lateral to the superior insertion of the temporalis muscle, which is elevated in a limited fashion. The inferior bony cut is preferably made just superior to the frontal sinus; however, transgression of the sinus can be fixed easily with the use of a rotational pericranial flap. The dura is opened in a horizontal incision inferiorly and the superior sagittal sinus is divided with medium vascular clips. The falx is incised and the anterior interhemispheric space is explored (Fig. 24-3).
Pterional Craniotomy
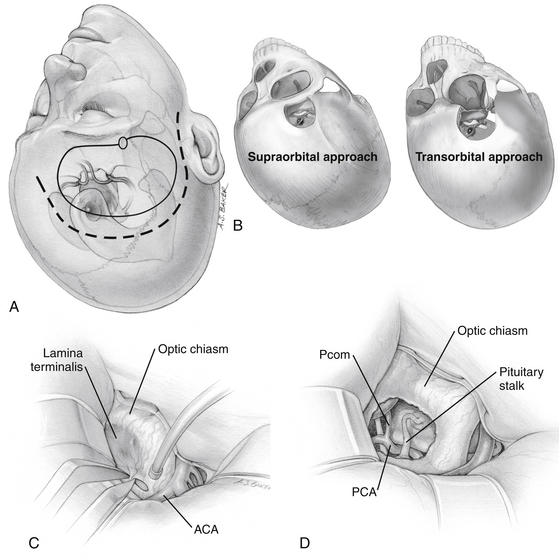
The traditional frontotemporal or pterional craniotomy is perhaps the most widely used approach for the treatment of craniopharyngiomas. Reduction of the sphenoid wing is a standard part of this approach because it improves exposure superiorly. Once the basal cisterns are opened, there are two major corridors for lesion resection: the subchiasmatic corridor between the two optic nerves and the opticocarotid corridor between the lateral aspect of the ipsilateral optic nerve and ICA (Fig. 24-4).
The supraorbital craniotomy and its transorbital variant provide a similar degree of exposure with somewhat limited soft tissue dissection (Fig. 24-4B). The temporalis muscle is mobilized to a much lesser degree. With the incision is linear within the eyebrow, there is little need for subcutaneous dissection. The bone flap, which averages 3 × 2 cm, provides adequate access to most lesions in the parasellar space. Care should be taken to avoid injury to the frontalis branch of the facial nerve along the lateral aspect of the incision. The use of frameless stereotactic guidance is recommended to prevent violation of the lateral recess of the frontal sinus. Reconstruction of the sinus from this approach is limited and may result in a transnasal CSF leak that can prove difficult to seal. In our practice, an extensive lateral recess of the frontal sinus is a contraindication for this approach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree