Fig. 8.1
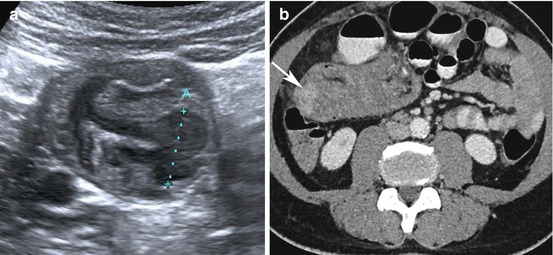
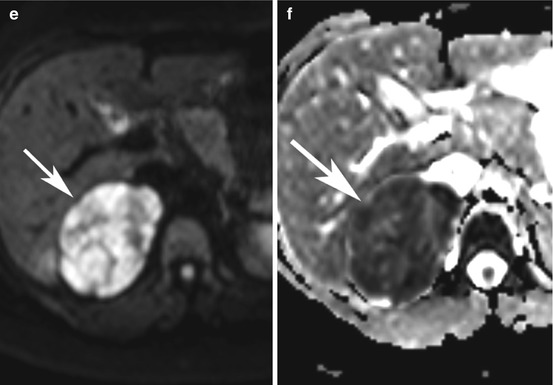
Adrenocortical carcinoma. A 39-year-old lady presented with Cushing’s syndrome and right-sided flank pain. (a) Ultrasound showed a large solid right suprarenal mass (between calipers) abutting the liver, with heterogeneous internal echoes. (b) Contrast-enhanced CT demonstrates a 7-cm-diameter heterogeneous hypodense right suprarenal lesion (arrow) abutting the right lobe of the liver. (c) Enhancing tumour extending into the IVC (white arrow). (d) Dynamic contrast-enhanced MRI showed slightly atypical gradual patchy and heterogeneous enhancement, with poor enhancement internally (white arrow) and avid rim enhancement (black arrow) after 5 minutes. Restricted diffusion is demonstrated by high signal on the b500 DWI (e) and low signal on the ADC map, (f) typical of malignancy (arrow). The lesion was excised via thoracotomy
8.3.1.1 CT
CT Imaging features suggestive of an ACC include a suprarenal mass measuring >4 cm with irregular margins, central intratumoural necrosis or haemorrhage, heterogeneous enhancement and fatty regions due to intracytoplasmic lipid, calcification (present in 30 %), invasion into adjacent structures and venous extension into the IVC and renal vein, more commonly found with right-sided lesions (Fig. 8.1) [15, 16]. Metastases to lymph nodes, lungs, liver and bone are frequently present on initial imaging.
In smaller lesions, measuring the density of adrenal lesions on both unenhanced and enhanced CT is a well-established tool that aids in differentiating benign from potentially malignant adrenal lesions. Adrenal adenomas have attenuation values of <10 Hounsfield units (HU) in 98 % of cases [17], in contrast to ACCs that will almost always have a HU of >10 on the unenhanced study. Following intravenous contrast administration, ACCs retain contrast for longer than a benign adenoma would, with an absolute percentage washout of <60 % and relative washout of <40 % at 10 min.
8.3.1.2 MRI
Lesions are typically iso- or very slightly hypointense to normal liver parenchyma on T1W images with high T1 seen in areas of haemorrhage. On T2W images lesions are usually hyperintense to liver with a heterogeneous appearance due to intratumoural cysts and haemorrhage. Lesions usually demonstrate restricted diffusion and avid enhancement throughout (Fig. 8.1d–f), with slow washout on delayed imaging; however, other enhancement patterns have been described, including peripheral mural-based enhancing nodules [18]. Chemical shift imaging (CSI), which is routinely performed to characterise adrenal nodules, typically shows no loss of signal in ACCs. However, in <30 % of functioning lesions, small irregular regions of signal dropout are demonstrated due to the scattered intracytoplasmic lipid found in these ACCs [18–20]. MR spectroscopy may be a potentially useful method of differentiating between lesions such as ACC, phaeochromocytoma, adrenal metastases and adrenal adenomas, with ACCs having a choline-creatine ratio of >1.20 and choline-lipid ratio of >0.38 and a 4.0–4.3 ppm/creatine ratio of >1.50 in one study [21].
8.3.2 Pheochromocytoma
These rare catecholamine-secreting tumours arise from the chromaffin cells of the adrenal medulla. Extra-adrenal pheochromocytomas can also be found in the paraspinal paraganglia, where they are termed paragangliomas, discussed separately below.
Pheochromocytomas are usually unilateral and benign; however, 10 % are bilateral and 10 % are malignant [22]. Bilateral pheochromocytomas are usually found in MEN II (50–80 %) and von Hippel-Lindau disease (40–80 %) and rarely in sporadic cases. Hypertension is the commonest presenting symptom; however, these lesions are rare and found in less than 1 % of hypertensive patients.
Pheochromocytomas often have relatively non-specific imaging features that overlap with other benign and malignant adrenal tumours on CT and MRI. Although generally large at presentation, there is marked variability in reported sizes, from 1.2 to 15 cm [23]. Lesion size may also affect the imaging characteristics of the tumour, with smaller lesions typically appearing more homogeneous and larger lesions heterogeneous [23], usually due to intratumoural haemorrhage [24].
8.3.2.1 Ultrasound
Ultrasound may locate moderately sized adrenal tumours, but it can be technically challenging and is operator dependent. The majority of lesions are completely solid, round and well-defined with a homogeneous echotexture which may be iso- or hyperechoic to liver and therefore similar to that of adjacent renal parenchyma [25]. However, approximately half of the solid tumours will appear complex, demonstrating a heterogeneous appearance with interspersed hyper- and hypoechoic regions. This correlates with macroscopic findings of intratumoural haemorrhage and necrosis respectively [25]. Cystic tumours appear as primarily anechoic lesions with posterior acoustic enhancement confirming their liquid property, correlating with old evolved haemorrhage and necrotic debris at pathology.
8.3.2.2 CT
An adrenal protocol scan (unenhanced, 60 s portal-venous phase and 15 min delayed post-contrast phase) is usually performed in order to quantify the intracellular lipid content of the lesion and to measure enhancement and washout characteristics (Fig. 8.2). Pheochromocytomas vary widely in appearance on non-contrast CT, with a range of densities from low to soft tissue. Although the majority will have an attenuation value of around 40–50 HU rarely HU may be much lower [17, 26]. Conversely higher-density lesions are found in cases of intratumoural haemorrhage. Approximately 10 % of lesions are reported to contain calcification, with nearly a quarter of symptomatic lesions being calcified. Following contrast administration, these tumours typically show avid enhancement of their solid components and may show homogeneous or heterogeneous enhancement depending on how complex their composition and relative amounts of internal haemorrhage and necrosis. Lesions show an absolute washout of less than 60 % and a relative percentage washout of less than 40 % at 15 min, or an absolute washout of less than 50 % and a relative percentage washout of less than 40 % at 10 min [27, 28]. However, variations can occur, and one study has shown pheochromocytomas to have similar washout patterns to adenomas [29].


Fig. 8.2
Pheochromocytoma (adrenal protocol CT). A 58-year-old female presented with refractory high blood pressure and palpitations. (a) Non-contrast CT demonstrates a 2.7 cm spherical solid lesion arising from the lateral limb of the right adrenal gland with a HU of 25 and no sign of fat or calcification (arrow). (b) It enhances heterogeneously with a HU of 92 on the first phase and 60 on the delayed phase. This gives the lesion a relative washout of 35 % and absolute washout of 48 %, in keeping with a non-adenomatous lesion. Histology proved this to be a phaeochromocytoma following surgical resection
8.3.2.3 MRI
Pheochromocytomas have been described as having a classical ‘light-bulb’ bright appearance on T2W, being brighter than fat and comparable with CSF [30]. They are typically isointense to muscle and hypointense to liver on T1W imaging. However, variations in appearance occur due to the often complex haemorrhagic and necrotic components. Haemorrhage of varying ages can have different signal characteristic on T1W images, with methaemoglobin in subacute haemorrhage resulting in high T1 and low T2 signal intensities. The rare cases that contain intracellular lipid will show loss of signal intensity on CSI (dual echo in and out of phase T1-weighted MRI) [31]. Pheochromocytomas typically demonstrate avid contrast enhancement post gadolinium administration, with a heterogeneous appearance when there are haemorrhagic, necrotic or cystic components [32].
8.3.3 Paraganglioma
These tumours (also known as extra-adrenal pheochromocytoma) arise from neuroectodermally derived paraganglionic cells anywhere within the body. Sympathetic paragangliomas typically secrete catecholamines, and common sites include the posterior mediastinum and abdominal paraaortic region including Zuckerkandl’s body [2]. Clinical presentation is typically with headache, palpitations and sweating, with potentially fatal cardiovascular effects including sudden myocardial infarction, malignant hypertension and cerebral haemorrhage [33]. Parasympathetic paragangliomas tend to be nonsecretory tumours, presenting insidiously with an enlarging palpable mass or pain related to the site of tumour. These lesions include aortic body tumours found in the aorticopulmonary window, as well as those found within the head and neck regions, including carotid body, jugular foramen and middle ear [33]. Most paragangliomas are solitary and sporadic; however, familial paraganglioma are recognised in 10 % of cases, including patients with succinate dehydrogenase B (SDHB) mutation where tumours can be multiple and have a higher rate of malignancy [34].
8.3.3.1 Head and Neck Paragangliomas
The carotid body is the commonest site, followed by jugular foramen, middle ear and along the distribution of the vagus nerve. Less common sites include the orbit, nasal cavity, nasopharynx, thyroid gland, pineal gland and cheek [35]. The carotid body, within the medial aspect of each carotid bifurcation, is the largest compact collection of paraganglia in the head and neck [33]. Carotid body tumours appear as a well-defined soft tissue mass within the carotid space of the infrahyoid neck, splaying the internal and external carotid arteries [36]. On CT, these lesions demonstrate avid homogeneous enhancement due to their marked hypervascularity (Fig. 8.3a–d) [33]. Occasionally, particularly in larger tumours, haemorrhage and necrosis will result in heterogeneous non-enhancing regions. On MRI they are typically of intermediate signal intensity on T1W and high on T2W imaging. A classical feature is the so-called ‘salt-and-pepper’ appearance, characterised by multiple punctate and serpiginous signal voids due to fast-flowing internal vessels, interspersed with high-signal regions due to slow flow or haemorrhage [37].




Fig. 8.3
SDHB mutation with multiple paragangliomas. A 57-year-old female presented with a pulsatile neck mass and persistently raised metanephrines. (a) Axial (arrow) and (b) coronal (arrow) reformatted arterial-phase CT demonstrates an avid arterially enhancing lesion at the left carotid bifurcation in keeping with a carotid body paraganglioma. (c) Post-contrast T1 fat-suppressed image showing the avidly enhancing paraganglioma at the carotid bifurcation, which slightly splays the bifurcation and extends posterior to the common and external carotid arteries. (d) Post-contrast fat-suppressed T1-weighted image shows a large avidly enhancing lesion within the left jugular foramen which has eroded and destroyed adjacent bone. Histology following surgical removal confirmed a glomus jugulotympanicum. (e) T2-weighted imaging through the thorax revealed a 3 cm hyperintense left paraspinal lesion (arrow). The lesion was not PET avid and was proven to be a paraganglioma on histology
8.3.3.2 Thoracic Paragangliomas
These constitute 1–2 % of all paragangliomas and occur predominantly in mediastinal compartments, with less common sites being the trachea, lung, heart and oesophagus. Aorticopulmonary paragangliomas, which are located within the anterior mediastinum, tend to present late as incidental lesions on chest radiographs, whereas the sympathetic posterior mediastinal lesions present earlier with symptoms related to catecholamine secretion [38]. On CT, lesions appear as well-enhancing typically homogeneous masses; however, in cases where there has been haemorrhage or cystic degeneration, patchy areas of low attenuation within the lesion may be seen [33]. Angiography demonstrates marked hypervascularity with multiple feeding vessels; preoperative embolisation may be considered in cases of bulky or surgically challenging tumours [39]. On MRI, lesions are homogeneous or heterogeneous intermediate signal intensity similar to liver parenchyma on T1W imaging, and are hyperintense compared to liver parenchyma on T2W images (Fig. 8.3e) [40].
8.3.3.3 Abdominal Paragangliomas
Retroperitoneal paragangliomas are most commonly seen in the tissues surrounding the aorta and inferior mesenteric artery. CT demonstrates a soft tissue mass with either homogeneous enhancement or central areas of low attenuation. Focal areas of high-density or punctate calcification due to haemorrhage may be seen [41]. On MRI lesions appear isointense or hypointense compared to liver on T1W imaging and are markedly hyperintense on T2W imaging [42].
8.4 Pancreatic Neuroendocrine Tumours (pNET)
Pancreatic endocrine tumours (pNETs) arise from the islet cells of Langerhans and include functioning tumours (insulinoma, gastrinoma, VIPoma, glucagonoma, somatostatinoma) and non-functioning tumours (including pancreatic polypeptidoma (PPoma) and tumours with no associated clinical syndrome).
Insulinomas are the commonest pNET. Diagnosis is based on clinical presentation and biochemical findings (Whipple’s triad). Lesions are typically benign (90 %), solitary and almost always intrapancreatic (>90 %), being equally distributed between the head, body and tail of pancreas [43, 44]. Due to their early presentation, they are often small at diagnosis, with 90 % measuring <2 cm and 40 % <1 cm in diameter [45–47].
Gastrinomas are the second commonest pNET. Clinical presentation is usually with the Zollinger-Ellison syndrome (ZES), due to gastrin-driven gastric acid secretion; the diagnosis is usually confirmed biochemically. The majority of cases are malignant; approximately 60 % of patients have hepatic metastases at presentation, and if associated with MEN1 there is an even higher incidence of malignancy [43, 48]. Regional nodal metastases are frequent, and bone metastases have been reported in approximately 30 % of cases [49]. The primary lesion is located within the ‘gastrinoma triangle’ in more than 90 % of cases (between the junction of the neck and body of the pancreas medially, the second and third parts of the duodenum inferiorly and the junction of the cystic and common bile ducts superiorly) [50]. Gastrinomas may be very small in size and are often extrapancreatic [51].
Other functioning pNETs are very rare (VIPomas, glucagonomas and somatostatinomas), frequently associated with MEN 1 and frequently malignant (between 50 and 80 %). They usually present late with a large mass and metastases. VIPomas may be extrapancreatic (in 20 %), predominantly in the retroperitoneal sympathetic chain and adrenal medulla [43, 50, 52]. Somatostatinomas are intrapancreatic (usually in head of pancreas) in over half of cases, the remainder being located in the duodenum and jejunum, usually in association with neurofibromatosis [54].
Non-functioning pNETS including PPoma and other tumours with no clinical syndrome tend to be sporadic and slow growing, presenting late as large tumours causing mass effect [45]. They usually lie in the pancreatic head, and thus presentation may be with biliary obstruction; 60–90 % are malignant [43, 52, 53]. These tumours may also be seen in von Hippel-Lindau syndrome.
8.4.1 Localisation of Pancreatic NETs on Imaging
A wide variety of imaging methods may be used for localising small functioning primary tumours, reflecting the difficulties encountered in detection. Local expertise also dictates the type of imaging used.
8.4.2 Ultrasound
8.4.2.1 Transabdominal Ultrasound
Transabdominal ultrasound (US) has a high specificity but low sensitivity for localising small pNETs in the range of 20–86 % [44, 55, 56]. As with most imaging techniques, sensitivity increases with the size of the lesion [56, 57].
Sonographic appearances are usually of a well-defined round mass, which is homogeneously hypoechoic in comparison to the relatively hyperechoic normal pancreatic tissue and may exhibit a hyperechoic halo. Hypervascularity is typical on Doppler imaging. Tumours that lie along the surface of the pancreas or in the duodenum are less conspicuous on ultrasound.
Contrast-enhanced ultrasound (CEUS) has shown a high specificity of 90–100 % in differentiating pancreatic ductal adenocarcinoma from other solid neoplastic masses, including pNETs which are typically hypervascular [58, 59]. CEUS has shown promising results in detecting small pNETs, with a sensitivity of 94 % and specificity of 96 % [60].
8.4.2.2 Endoscopic Ultrasound
Endoscopic ultrasound (EUS) has been reported to have a high sensitivity (90 %, range 77–100 %) for the detection of small functioning pNETs [61–64].
EUS with a high-frequency probe (7.5–10 mHz) results in superior image resolution due to the close proximity of the transducer to the pancreas. Contrast enhancement may also be used. The probe is positioned within the stomach for visualisation of the pancreatic body and tail and in the duodenum for the head and duodenum [63, 65]. The advantages include localisation of small tumours, particularly in the pancreatic head which may be difficult to palpate at surgery; multiple tumours, in cases of MEN 1; tumours arising in the duodenal wall; and accurate depiction of the relations between tumour and vascular and biliary structures. FNAC can also be undertaken which has a close correlation to the final histology [66, 67].
EUS can identify regional nodal enlargement, but it cannot fully assess the liver. It is technically challenging and highly specialised, therefore not widely available. Detection of lesions in the tail or extrapancreatic area can be challenging. It also may not be suitable for all patients, for example, where there is duodenal scarring in ZES.
8.4.2.3 Intraoperative Ultrasound
Intraoperative ultrasound (IOUS) has similar advantages to EUS and may improve the intraoperative sensitivity to 92–97 % for identifying small lesions in the head and multiple lesions and is a useful adjunct to palpation [55, 68]. It has been shown to change operative management by identifying multiple gastrinomas or by demonstrating the malignant nature of a lesion in up to 10 % of ZES cases [69]. IOUS has the advantage over EUS of being able to assess the liver, although it is not as sensitive as surgical palpation in detecting extrapancreatic lesions. Disadvantages of the technique include increasing the time and complexity of operation, as complete mobilisation of the pancreas is required, specialist experience for performing and interpreting the scan and poor sensitivity in detecting extrapancreatic/duodenal lesions.
8.4.2.4 CT
Multidetector CT is the most widely used diagnostic tool for the localisation and staging of pNETs.
CT Technique
The patient should be fasted to ensure that the stomach and duodenum are empty. The stomach is then distended with water just prior to scanning, and an IV antiperistaltic agent is administered. An initial pre-contrast scan is performed at the level of the pancreas followed by biphasic post-contrast scanning. Depending on the contrast medium used, around 150 ml should be administered at a rate of 3–5 ml/s. Arterial-phase scanning should be performed either by bolus tracking or after a delay of 25–30 s, followed by portal-venous scanning after 60–70 s with a section thickness ≤5 mm, and the entire liver should be included in both phases. The images are then reconstructed to 1–2 mm in slice thickness, and coronal or sagittal reformats should be reviewed. Images should also be viewed on narrow window settings in order to augment the difference between the enhancing tumour and pancreatic tissue [70, 71].
CT Appearance
Functioning pNETs are usually small and subtle at presentation, appearing isodense to normal pancreatic tissue on pre-contrast images. The majority of pNETs are hypervascular and will only be seen after IV contrast medium. Some tumours are seen more easily on arterial phase and others on portal phase images (Fig. 8.4) [72–75]. Rarely, insulinomas may be hypovascular or cystic and will therefore appear hypodense to the surrounding pancreas (Fig. 8.5) [76]. Cystic pNETs are usually benign and non-functioning; they cannot be reliably differentiated from other cystic pancreatic neoplasms on imaging alone [76, 77]. In patients with a suspected gastrinoma, particular attention should be given to the ‘gastrinoma triangle’ and duodenal wall, where lesions are commonly found (Fig. 8.6).




Fig. 8.4
Insulinoma in neck of the pancreas. An 81-year-old female presented with hyperinsulinaemia. (a) Arterial-phase CT shows an 18 mm arterially enhancing lesion in the neck of the pancreas (arrow); (b) portal-venous-phase CT shows that the lesion remained avidly enhancing (arrow)

Fig. 8.5
Cystic pancreatic NET. A 61-year-old female. (a) Incidental 16 mm lesion in the tail of the pancreas which demonstrated increased vascularity peripherally, suggestive of a cystic NET (arrow). (b) Gallium PET demonstrated avidity in keeping with a NET

Fig. 8.6
Duodenal gastrinoma. A 49-year-old gentleman presented with Zollinger-Ellison syndrome. (a) Arterial-phase CT shows generalised gastric rugal hypertrophy consistent with hypergastrinaemia. (b) A small hypervascular lesion on the posterior wall of the third part of the duodenum was identified consistent with a gastrinoma, shown here on axial CT (white arrow)
Diagnostic Performance of CT
Multidetector CT with multiplanar thin reformats has improved the sensitivity for detection of the primary tumour, particularly insulinomas, with one study reporting a sensitivity of 94 % on MDCT alone and 100 % when combined with EUS [82]. Detection is directly related to tumour size, with significant difficulty in detecting tumours <1 cm, detection of 30 % of tumours between 1 cm and 3 cm and 95 % of tumours >3 cm [45, 50, 78]. Tumours within the head and body have a higher detection rate than those in the tail [78, 79]. Small duodenal tumours <1 cm are often missed on CT, and CT sensitivity for the detection of extrahepatic and extrapancreatic gastrinomas, which are often small at presentation, is only 30–50 % [51, 78].
8.4.2.5 Magnetic Resonance Imaging
Advances in MR technology have improved the diagnostic performance in lesion detection [75, 80, 81]. A sensitivity of 94 % for pancreatic lesions, but less for extrapancreatic lesions, has been reported [80, 82]. Although angiography has been reported to be more sensitive for identifying the primary tumour, MR has a higher sensitivity than angiography and CT for regional metastatic disease [83]. Tumour detection increases with tumour size, and small multiple tumours in patients with MEN 1 remain difficult to detect.
Optimal technique is needed for high sensitivity as degradation of images due to artefact leads to difficulty in detection. The sequences should include axial T1W, T2W, T1 fat-suppressed spin echo and gradient echo, dynamic contrast enhancement and diffusion-weighted imaging (DWI).
Lesions are usually of low signal intensity on T1W and high on T2W sequences in relation to normal pancreatic tissue, often appearing most conspicuous on the fat-suppressed T1W images (Fig. 8.7) [80, 82]. Rarely, tumours contain a high collagen or fibrous tissue content, with low T2 signal intensity [75]. Following IV gadolinium, characteristic marked homogeneous enhancement is demonstrated. Cystic lesions may demonstrate rim enhancement [80]. Liver-specific contrast agents have been reported to improve detection of liver metastases [84, 85].


Fig. 8.7
Multiple pancreatic NETs in patient with MEN1. MRI evaluation. (a) T1W fat saturated demonstrates a lesion in the uncinate process; (b) T2W fat-saturated images shows a high-signal-intensity lesion arising from the tail of the pancreas
DWI is an MRI technique that measures water diffusivity in biological tissues, generating the quantifiable apparent diffusion coefficient (ADC). Small pancreatic NETs may be sensitively detected due to high contrast resolution, and the ability to distinguish benign from malignant lesions is under investigation [86].
8.4.2.6 Angiography
Diagnostic angiography is used in selected cases in specialist centres and is combined with venous sampling (see below). Detailed assessment of the vasculature includes selective catheterisation of all the branches of the coeliac and superior mesenteric arteries. Primary tumours and liver metastases are seen with high sensitivity as a well-defined blush in the capillary and early venous phase. Diagnostic difficulties can arise if a tumour is very small, multiple, or hypovascular or when a lesion lies adjacent to a loop of bowel or spleen and the blush is not separately visible. False positives arise from the blush of a splenunculus or normal pancreas or bowel.
8.4.2.7 Transhepatic Portal-Venous Sampling
Transhepatic portal-venous sampling (TSPVS) involves percutaneous catheterisation of a portal-venous branch. Blood is sampled from the splenic vein, superior and inferior mesenteric veins, pancreatic veins and portal trunk, with the principle that one of these veins will be draining the tumour and therefore contain high concentrations of the secreted hormone. This method is only useful for functioning tumours, although false-negative results occur if hormone secretion is intermittent.
8.4.2.8 Arterial Stimulation and Venous Sampling
Arterial stimulation venous sampling (ASVS) combines simultaneous hepatic venous sampling with selective arterial injection of a pancreatic secretagogue (usually calcium gluconate), a technique that is less invasive than THPVS. Following injection of the secretagogue, hepatic venous sampling is performed every 30 s for 2 min. A two- to threefold increase in the level of hormone indicates the regional presence of tumour. This technique is most useful in cases where a functional tumour remains occult on other imaging modalities. Reported sensitivities are as high 93 %, and the stimulation technique improves the sensitivity of angiography alone [50].
8.5 Gastrointestinal Neuroendocrine Tumours
Gastrointestinal NETs (previously known as carcinoids) are traditionally classified according to their site of origin. The secretory products and therefore the clinical manifestations and immunohistochemical staining patterns are similar for tumours arising from different anatomical sites. ‘Foregut carcinoids’ include NETs arising from the thymus, bronchus, gastric or duodenal mucosa and pancreas; ‘midgut carcinoids’ arise in the jejunum, ileum and proximal colon; while ‘hindgut’ NETs arise in the distal colon and rectum [87]. The most commonly reported sites are the bronchus (32.5 %), jejuno-ileum (20 %), appendix (8 %) and rectum (10 %). They are usually highly vascular tumours, resulting in their characteristic brightly enhancing appearances on imaging.
8.5.1 Bronchial Neuroendocrine Tumours
Bronchopulmonary NETs arise from Kulchitsky cells, neuroepithelial bodies or pluripotential bronchial epithelial stem cells in the bronchial epithelium [88, 89]. Tumours are graded by the World Health Organization according to their malignant potential, from low grade typical carcinoid to small-cell carcinoma [90].
Imaging features vary depending on whether the tumour is located in the airways of the central/middle third of the lung (80 % of cases) or in the peripheral airways [91]. Plain radiography may demonstrate a well-demarcated round or ovoid mass, which is often notched [92]. Centrally located tumours will appear as a central mediastinal or hilar mass, usually measuring 2–5 cm in diameter. These lesions may result in airways obstruction, with recurrent infection and/or lobar collapse [92]. However, as they are often small, CT scanning is more sensitive for detection. Lesions within the bronchial lumen typically brightly enhance (which can mimic a vessel), usually with both an intra- and extraluminal component being visible. Distal collapse or air trapping may be seen due to ball-valve obstruction of the bronchial lumen. Peripheral bronchial NETs are seen in 20 % of cases, appearing as solitary pulmonary nodules, typically round or ovoid with a smooth or lobulated border, commonly with punctate or diffuse calcification. Cavitation and hilar adenopathy and mediastinal invasion may be seen in the more aggressive histological subtypes [92]. In patients with occult ectopic ACTH secretion, a bronchial NET is the commonest source; however, these can be difficult to identify. When a pulmonary lesion is suspected but not seen on CT, MR may aid localisation. Bronchial carcinoids have high signal intensity on T2W and short tau inversion recovery (STIR) images, allowing differentiation between a small mass and the pulmonary vasculature [93].
8.5.2 Thymic Neuroendocrine Tumours
NETs of the thymus are extremely rare, typically presenting with an anterior mediastinal mass. Thought to arise from thymic cells of neural crest origin (Kulchitsky cells), these tumours have similarities to bronchial NETs with a range of tumour differentiation and behaviour. Approximately 50 % of tumours are functioning, with about 40 % presenting with Cushing’s syndrome, in which case the mass is usually smaller than those patients presenting with non-functioning tumours [94]. Patients with non-functioning tumours (Fig. 8.8) usually present with symptoms related either to mass effect and/or local invasion. Lesions may be heterogeneous on CT (Fig. 8.9) and may show calcification. Assessment of the SVC for obstruction is vital [95, 96]. Invasive disease as evidenced by extension into the pleura, pericardium, great vessels or regional lymph nodes is also frequently seen in this group of patients [97]. Bilateral adrenal hyperplasia may be present in those with functioning ACTH-secreting tumours [95]. Metastatic disease in lungs, liver and bone, which may be sclerotic, may also be present at the time of diagnosis [96–99].



Fig. 8.8
Neuroendocrine tumour of the thymus. A 37-year-old man presented with recurrent chest pain for several years and a previous episode of pericarditis. Chest radiograph showed a large lobulated mediastinal mass. The patient proceeded to a CT scan, which demonstrates a 16 cm heterogeneously enhancing anterior mediastinal mass (star), displacing the aorta and pulmonary trunk posteriorly. Functional imaging in the form of gallium-68 DOTATAE showed the lesion to be somatostatin receptor positive. The lesion was unsuitable for resection, and the patient underwent lutetium-177 therapy

Fig. 8.9
Thymic NET. (a) A 77-year-old male presented with chest sepsis. CT chest revealed an incidental 5 cm lobulated, heterogeneously enhancing anterior mediastinal mass with hypodense regions suggestive of necrosis and tiny calcific foci. (b) F-18 FDG PET shows metabolic activity within the lesion. Histology showed neuroendocrine carcinoma, with an atypical marker profile
8.5.3 Gastric Neuroendocrine Tumours
Gastric NETs account for 0.3 % of gastric neoplasms but 11–41 % of all GI NETs [100]. Gastric, duodenal and colorectal neuroendocrine tumours are usually diagnosed by endoscopy with EUS to determine depth of invasion and regional nodal status and for fine-needle aspiration [62]. CT is predominantly done to detect regional and distant metastatic disease. Administration of an antiperistaltic agent and a negative oral contrast agent (water) may also improve detection of the gastric NETs. CT should include an arterial phase acquisition at 25–30 s following the injection of contrast agent at a rate of 4–5 ml/s, followed by a delayed portal-venous phase at 60 s.
Type I gastric NETs, associated with atrophic gastritis, are poorly seen on CT and MR, being multicentric and typically <1 cm in size. The disease is almost always benign, with metastases present in only 2 % of cases. Type II gastric NETs are associated with MEN-1. There are typically multiple lesions within the gastric wall, which is thickened due to the association with the ZES. There is an increased tendency to metastasise to regional lymph nodes, although the prognosis is generally good [101]. Type I and type II gastric NETs may appear similar to other gastric polyps as numerous enhancing submucosal lesions [102]. Type III sporadic gastric NETs are usually solitary, large lesions with an irregular contour and diffuse margins that may ulcerate. Local invasion with extension into the surrounding gastric fat and metastases are common at presentation.
8.5.4 ‘Midgut’ Neuroendocrine Tumours
Midgut NETs arise beyond the ligament of Treitz to the level of the mid-transverse colon. They are the commonest primary malignant tumour of the small bowel, as well as being the commonest location of all GI NETs [103]. Patients present with abdominal pain, usually with associated colic and diarrhoea, or with symptoms of obstruction that may be due to the primary tumour or due to intussusception (Fig. 8.10). Tumour-associated desmoplastic mesenteric fibrosis due to the local production of fibrogenic agents results in tethering and kinking of the small bowel mesentery that can also result in obstruction. Metastases are often present at diagnosis, most frequently to the liver, bone and lung [104–106]. The incidence of metastases is dependent on tumour size [104, 106], with tumours <1 cm having metastases in 15–25 % of cases, 1–2 cm in size 58–80 % of cases and >2 cm in over 70 % of cases.