Fig. 6.1
Top row: Five healthy participants asked to imagine playing tennis in the fMRI scanner (Adapted from [6]). In all five cases, significant activity was observed in the premotor cortex, indicating that they had understood the instruction and were responding by carrying out the appropriate type of mental imagery, that is, following a command. Bottom row: Formally identical responses in five patients who behaviorally meet the clinical criteria for a diagnosis of vegetative state (Adapted from Owen et al. [14] (patient 5) and [15] (patients 1–4)) confirming that, in spite of an inability to respond physically, these patients can still demonstrate command following by modulating their cortical fMRI activity. Such responses are observed in approximately 17 % of vegetative patients
After a severe brain injury, when the request to move a hand or a finger is followed by an appropriate motor response, the diagnosis can change from vegetative state (no evidence of awareness) to minimally conscious state (some evidence of awareness). By analogy then, if the request to activate, say, the supplementary motor area of the brain by imagining moving the hand is followed by an appropriate brain response, we should give that response the very same weight [16–18]. Skeptics may argue that brain responses are somehow less physical, reliable, or immediate than motor responses but, as is the case with motor responses, all of these arguments can be dispelled with careful measurement, replication, and objective verification [6, 14, 15, 16, 19, 20, 21]. For example, if a patient who was assumed to be unaware raised his or her hand to command on just one occasion, there would remain some doubt about the presence of awareness given the possibility that this movement was a chance occurrence, coincident with the instruction. However, if that same patient were able to repeat this response to command on ten occasions, there would remain little doubt that the patient was aware. By the same token, if that patient was able to activate his or her supplementary motor area in response to command (e.g., by being told to imagine hand movements) and was able to do this on every one of ten trials, we would have to accept that this patient was consciously aware. Like most neuroimaging investigations, replication of this sort was inherent in both of the studies described above [14, 15], because the statistically significant results depended on multiple, similar responses being exhibited across repeated trials.
Monti et al. [15] employed the same general principle – that is, using the neural signature of active mental rehearsal to signify awareness – to show that communication of “yes” and “no” responses was possible with fMRI. Thus, three “yes” and “no” responses were decoded from each of 16 healthy participants with 100 % accuracy using only their real-time changes in the supplementary motor area (during tennis imagery) and the parahippocampal place area (during spatial navigation). Moreover, in one traumatic brain injury patient, who had been repeatedly diagnosed as vegetative over a 5-year period, similar questions were posed and successfully decoded using the same approach [15]. Indeed, this patient was able to convey biographical information that was not known to the experimenters at the time (but was verified as factually correct) such as his father’s name and the last place that he had visited on vacation before his accident 5 years earlier. In contrast, and despite a reclassification to minimally conscious state following the fMRI scan, it remained impossible to establish any form of communication with this patient at the bedside.
An obvious application for approaches of this sort is to begin to involve some of these patients in the decision-making processes involved in their own therapeutic care and management. To date, this has only been achieved successfully in one patient, who had been repeatedly diagnosed as vegetative for 12 years following a traumatic brain injury [16]. The patient was a male who, in December 1999 and at the age of 26, had suffered a severe closed head injury in a motor-vehicle accident. On admission to hospital he had a Glasgow Coma Scale [22] score of 4, meaning that he was unable to open his eyes or produce any sound and his only response was extension to painful stimulation. Over the next 12 years, the patient was assessed regularly by experienced neurologists and multidisciplinary teams and throughout this period his behavior remained consistent with the internationally accepted criteria for the vegetative state. Indeed, over one 14-month period in 2011–2013, a total of 20 standardized behavioral assessments were performed by a multidisciplinary team, at different times of the day and in different postural positions, using the Coma Recovery Scale-Revised [23], and his diagnosis was unchanged throughout. In February 2012, 12 years and 2 months after his accident, the patient was first scanned using the fMRI mental imagery approach described above [14, 15]. The patient was able to provide correct answers to multiple externally verifiable questions, including his own name, his whereabouts, the name of his personal support worker (who he had only encountered in the years following his accident), the current date, and other basic factual information (e.g., whether a banana is yellow). Two non-verifiable questions were then posed, including one pertaining to his care preferences (e.g., whether he liked watching (ice) hockey games on TV) and another to details about his current clinical condition (e.g., whether he was in any physical pain). Within the time constraints of the scanning visits, the majority of responses to these questions were verified in independent sessions that posed the reverse questions (e.g., “Is your name Mike?” vs. “Is your name Scott?”). In total, answers to 12 different questions were obtained across several sessions, despite the fact that the patient remained entirely physically nonresponsive at the bedside [16].
Although techniques like the ones described above require that the patient engages in rather specific types of mental imagery (playing tennis or moving from room to room through a house), that is not really the main point that allows consciousness to be detected and communication to occur. All that is required to detect consciousness is a reliable indicator that a patient can turn his or her attention to a specific scenario, because this then serves as a “neural proxy” for a physical “response to command.” By extension, if it can be shown that the patient can turn his or her attention to two separate scenarios, then communication is possible because those two separate scenarios can be linked to “yes” responses and “no” responses, respectively. Thus, mental imagery is not necessary at all, but serves as a simple vehicle for guiding a patient’s attention one way or another.
A related and possibly simpler approach to detecting covert awareness after brain injury, therefore, is to target processes that require the willful adoption of “mind-sets” in carefully matched (perceptually identical) experimental and control conditions. For example, Monti et al. [24] presented healthy volunteers with a series of neutral words and alternatively instructed them to just listen, or to count, the number of times a given word was repeated. As predicted, the counting task revealed the frontoparietal network that has been previously associated with target detection and working memory. When tested on this same procedure, a severely brain-injured patient produced a very similar pattern of activity, confirming that he could willfully adopt differential mind-sets as a function of the task conditions and could actively maintain these mind-sets across time. These covert abilities were entirely absent from his documented behavioral repertoire. As in the tennis/spatial navigation examples described above, because the external stimuli (a series of words) were identical in the two conditions, any difference in brain activity observed cannot reflect an “automatic” brain response (i.e., one that can occur in the absence of consciousness). Rather, the activity must reflect the fact that the patient has performed a particular action (albeit a “brain action”) in response to the stimuli on one (but not the other) presentation; in this sense, the brain response is entirely analogous to a (motor) response to command and should carry the same weight as evidence of awareness.
Naci and colleagues [20, 21] took this general principle even further and developed a novel tool for communicating with nonresponsive patients based on how they selectively directed their attention to sounds while in the fMRI scanner. It is well established that selective attention can significantly enhance the neural representation of attended sounds [25], although most previous studies have focused on group-level changes rather than individual responses that are crucial for work with (individual) brain-injured patients. In their first study [20], 15 healthy volunteers answered questions (e.g., “Do you have brothers or sisters?”) in the fMRI scanner, by selectively attending to the appropriate word (“yes” or “no”), which was played to them auditorily, interspersed with “distractor” stimuli (digits 1–9). Ninety percent of the answers were decoded correctly based on activity changes within the attention network of the brain. Moreover, the majority of volunteers conveyed their answers with less than 3 min of scanning, which represents a significant time saving over the mental imagery methods described above [6, 14]. Indeed, a formal comparison between the two approaches revealed improved individual success rates and an overall reduction in the scanning times required to correctly detect responses; 100 % of volunteers showed significant task-appropriate activity to the selective attention task, compared to 87 % to the motor imagery. This result is consistent with previous studies showing that a proportion of healthy volunteers do not produce reliable brain activation during mental imagery tasks [6].
In a follow-up study, Naci and Owen [21] used the same approach to test for residual conscious awareness and communication abilities in three behaviorally nonresponsive, brain-injured patients. As in the previous study of healthy participants, the patients had to either “count” or “relax” as they heard a sequence of sounds. The word count at the beginning of the sequence instructed the patient to count the occurrences of a target word (yes or no), while the word relax instructed them to relax and ignore the sequence of words. Reliable activity increases in the attention network of the brain after the word count relative to the word relax was taken as evidence of command following. All three patients (two of whom were diagnosed as being in a minimally conscious state and one as being in a vegetative state) were able to convey their ability to follow commands inside the fMRI scanner by following the instructions in this way. In stark contrast, extremely limited or a complete lack of behavioral responsivity was observed in repeated bedside assessments of all three patients. These results confirm that selective attention is an appropriate vehicle for detecting covert awareness in some behaviorally nonresponsive patients who are presumed to mostly or entirely lack any cognitive abilities whatsoever.
In a following series of scans, communication was attempted in two of the patients. The communication scans were similar to those in the command-following scan, with one exception. Instead of an instruction (count or relax), a binary question (e.g., “Is your name Steven?”) preceded each sound sequence. Thus, each patient then had to willfully choose which word to attend to (count) and which to ignore, depending on which answer he wished to convey to the specific question that had been asked. Using this method, the two patients (one diagnosed as minimally conscious state and one diagnosed as vegetative state) were able to use selective attention to repeatedly communicate correct answers to questions that were posed to them by the experimenters [21]. In the absence of external cues as to which word the patient was attending to, the functional brain activation served as the only indicator of the patient’s intentions and, in both cases, led to the correct answers being decoded. For example, when asked, “Are you in a supermarket?” one patient showed significantly more activation for “no” than “yes” sequences in a network of brain areas that had been previously activated when that patient was focusing attention on external cues (Fig. 6.2). Conversely, when asked, “Are you in a hospital?,” the patient showed significantly more activation for “yes” than “no” sequences in the same regions. Despite his diagnosis (vegetative state for 12 years), the fMRI approach allowed this patient to establish interactive communication with the research team in 4 different fMRI sessions. The patient’s brain responses within specific regions were remarkably consistent and reliable across two different scanning visits, 5 months apart, during which the patient maintained the long-standing vegetative state diagnosis. For all four questions, the patient produced a robust neural response and was able to provide the correct answer with 100 % accuracy. The patient’s brain activity in the communication scans not only further corroborated that he was, indeed, consciously aware but also revealed that he had far richer cognitive reserves than could be assumed based on his clinical diagnosis. In particular, beyond the ability to pay attention, these included autobiographical knowledge and awareness of his location in time and space.
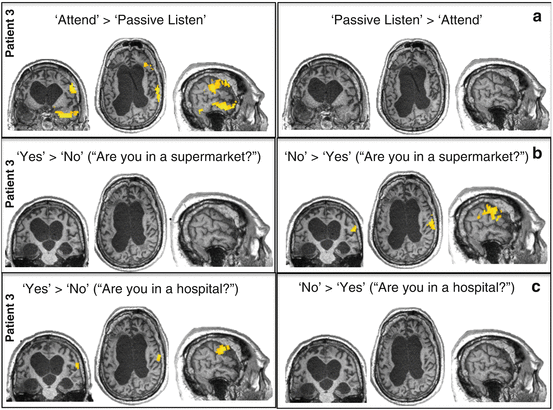

Fig. 6.2
Command-following (a) and communication (b, c) scans in patient 3, clinically diagnosed as being in a vegetative state. Brain activity is overlaid on the patient’s native anatomic volume. The opposite directions of each contrast (i.e., a > b or b > a) are shown on the left and right sides of each panel. (a) The command-following scan also served to localize the brain foci of attention unique to the patient. (b, c) Selective attention to the answer word (either yes or no) during each communication scan was investigated within these regions. Attention to the answer in each question (b, no; c, yes) significantly activated the precentral or motor region
These types of approaches all illustrate a paradigmatic shift toward the use of active (e.g., willful) tasks in the assessment of covert awareness after serious brain injury. What sets such tasks apart is that the neural responses required are not produced automatically by the eliciting stimulus, but rather, depend on time-dependent and sustained responses generated by the participants themselves. Such behavior (albeit neural “behavior”) provides a proxy for a motor action and is, therefore, an appropriate vehicle for reportable awareness [26].
Nevertheless, while “active” paradigms have proven themselves to be an effective means for assessing residual awareness in some nonresponsive patients, it remains likely that many patients will lack the necessary cognitive resources for carrying out these tasks in the scanner and will therefore fail to exhibit signs of awareness even when it may exist. To address this issue, recent efforts have focused on developing new methods for detecting awareness in the absence of an explicitly willed task. Naci and colleagues [27] used a richly evocative stimulus – a highly suspenseful movie – to capture attention naturally in the absence of structured instruction. They asked whether a common neural basis can account for how different individuals form similar conscious experiences and, if so, whether it could be used to interpret those experiences without recourse to self-report in behaviorally nonresponsive patients. They reasoned that executive function, in particular, might provide an empirical window by which the cognitive aspect of human conscious experience can be quantified. By their very nature, engaging movies are designed to give viewers a shared conscious experience driven, in part, by the recruitment of similar executive processes, as each viewer continuously integrates their observations, analyses, and predictions while filtering out any distractions, leading to an ongoing involvement in the movie’s plot.
When healthy participants viewed a highly engaging short movie by Alfred Hitchcock – the so-called Master of Suspense – in the fMRI scanner, they displayed highly synchronized brain activity in supramodal frontal and parietal regions, which support executive function [28, 29]. The movie’s executive demands, assessed quantitatively with a dual-task procedure [30] by an independent group, predicted activity in frontal and parietal regions of the healthy participants, who had watched the movie without a secondary task in the scanner. Importantly, the movie’s suspense ratings, provided by a third independent healthy group, demonstrated that individual participants had a similar qualitative experience of the movie, which also predicted activity in the frontal and parietal regions. Together, these results suggested that the movie’s executive demands drove brain activity in frontal and parietal regions and, further, that the synchronization of this activity across individuals underpinned their similar experience. By extension, the degree to which each individual’s frontoparietal brain activity could be predicted from the rest of the group’s represented a reliable neural index of how similar his or her cognitive experience was to the others’.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








