Chapter 2 Development of the Nervous System
The focus in this chapter is on the events that lead to the shape of the CNS and the configuration of its major components. There is clearly much more to building a nervous system than this—neurons must proliferate in enormous numbers, migrate from their places of birth to their final destinations, and establish appropriate connections with other neurons. Some of these aspects are touched on in Chapter 24.
The Neural Tube and Neural Crest Give Rise to the Central and Peripheral Nervous Systems
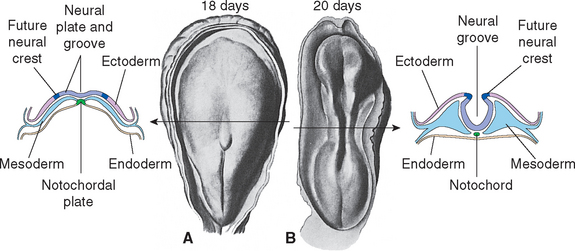
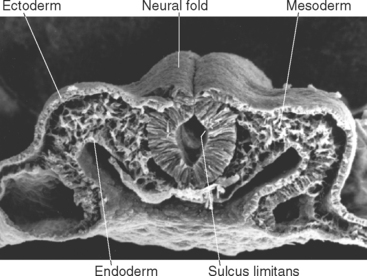
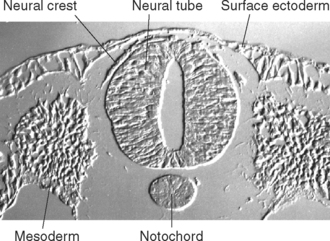
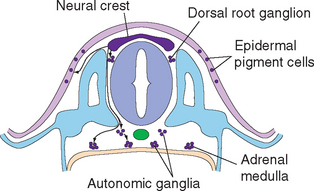
During the third week of embryonic development, in response to chemical signals released by the underlying midline mesoderm, a longitudinal band of ectoderm thickens to form the neural plate. Shortly thereafter, the neural plate begins to fold inward, forming a longitudinal neural groove in the midline flanked by a parallel neural fold on each side (Figs. 2-1 and 2-2). The neural groove deepens, and the neural folds approach each other in the dorsal midline. At the end of the third week the two folds begin to fuse midway along the neural groove at a level corresponding to the future cervical spinal cord, starting the formation of the neural tube (Fig. 2-3). Additional sites of fusion soon appear, the regions in between zip up, and the entire neural tube is closed before the end of the fourth week. This process is referred to as primary neurulation. As the neural tube closes, it progressively separates from the ectodermal (i.e., skin) surface and becomes enclosed within the body, leaving behind groups of cells from the crest of each neural fold (Fig. 2-4). These neural crest cells develop into a variety of cell types (Fig. 2-5), including the sensory neurons of the ganglia of spinal nerves and some cranial nerves,* the postganglionic neurons of the autonomic nervous system, and the Schwann cells and satellite cells of the peripheral nervous system (PNS). The neural tube develops into virtually the entire CNS; its cavity becomes the ventricular system of the brain.
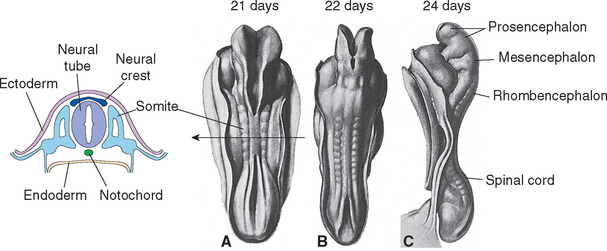
Figure 2-3 Neural tube closure during the fourth week. A, Neural folds begin to fuse at the cervical level of the future spinal cord at about day 21; the total length of the neural tube at this time is about 2.5 mm. B, This and additional areas of fusion expand rapidly in both rostral and caudal directions. C, By about day 24, the rostral end of the neural tube has closed; the caudal end will close about 2 days later. Even before the neural tube has finished closing, local enlargements (the primary vesicles) and bends begin to appear. The notochord is the forerunner of the skeletal axis, helping to form the vertebral column. The mesodermally derived somites, adjacent to the neural tube, go on to form most of the vertebral column, as well as segmental structures such as skeletal muscle and dermis corresponding to spinal cord segments (see Chapter 10).
(From Arey LB: Developmental anatomy, ed 4, Philadelphia, 1941, WB Saunders.)
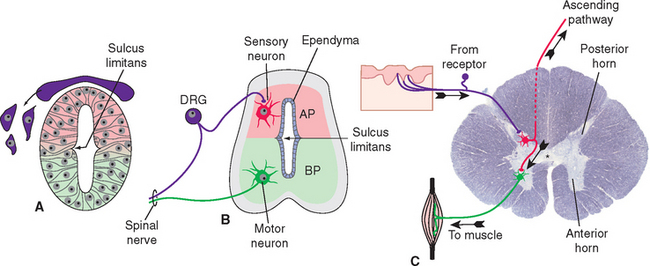
The Sulcus Limitans Separates Sensory and Motor Areas of the Spinal Cord and Brainstem
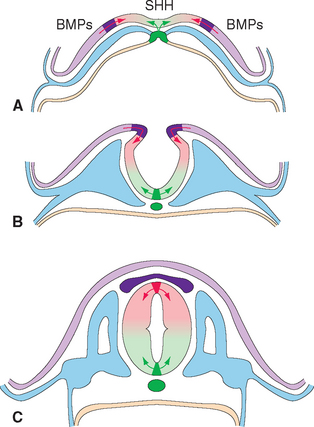
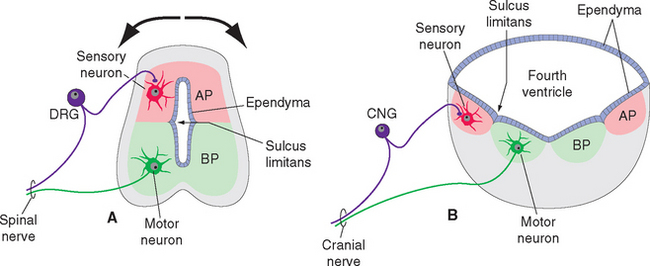
The ectoderm near what will become the dorsal surface of the neural tube, and the mesodermal notochord near the ventral surface, produce different signaling molecules early in development. The opposing concentration gradients of these signaling molecules induce distinctive patterns of subsequent development in these two regions of the neural tube (Fig. 2-6). This becomes morphologically apparent during the fourth week, when a longitudinal groove (the sulcus limitans) appears in the lateral wall of the neural tube, separating it into a dorsal half and a ventral half throughout the future spinal cord and brainstem. The gray matter of the dorsal half forms an alar plate and that of the ventral half forms a basal plate (Figs. 2-2 and 2-7). This is a distinction of great functional importance because alar plate derivatives are concerned primarily with sensory processing, whereas motor neurons are located in basal plate derivatives. In the adult spinal cord, even though the sulcus limitans is no longer apparent, the central gray matter can be divided into a posterior horn and an anterior horn on each side (Fig. 2-7C). The central processes of sensory neurons (derived from neural crest cells) end mainly in the posterior horn, which contains most of the cells whose axons form ascending sensory pathways. In contrast, the anterior horn contains the cell bodies of somatic and autonomic motor neurons, whose axons leave the spinal cord and innervate skeletal muscles and autonomic ganglion cells. The same distinction between sensory alar plate derivatives and motor basal plate derivatives holds true in the brainstem, as discussed briefly in this chapter and in more detail in Chapter 12. (The sulcus limitans does not extend beyond the brainstem, although concentration gradients of the same signaling molecules induce other distinctive dorsal-ventral patterns of development in the cerebrum. Hence the alar-basal plate distinction is not useful for cerebral structures, even though some deal primar-ily with sensory processes and others with motor processes.)
The Neural Tube Has a Series of Bulges and Flexures
The neural tube is never a simple, straight cylinder. Even before it has completely closed, bulges begin to appear in the rostral the neural tube in the region of the future brain, and bends begin to appear as well (Fig. 2-3C).
There Are Three Primary Vesicles
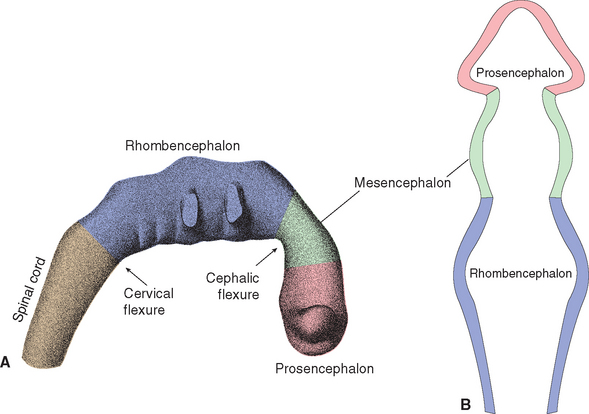
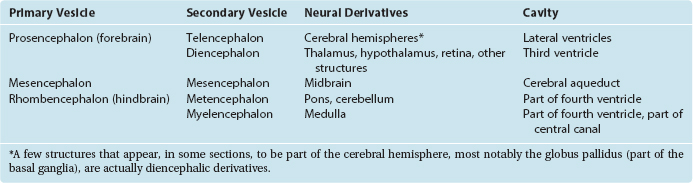
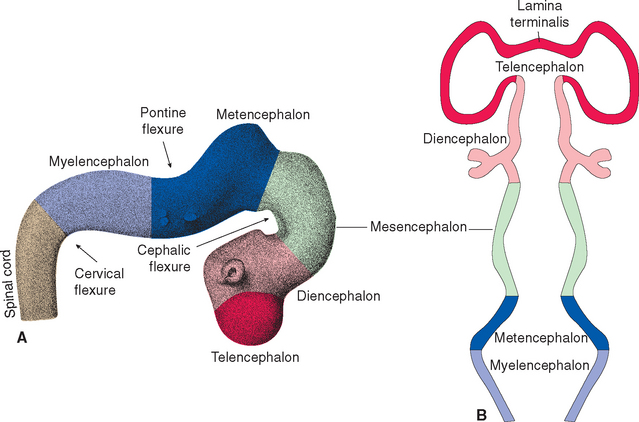
During the fourth week, three bulges, or vesicles, are apparent and are referred to as the primary vesicles (Fig. 2-8). From rostral to caudal, these are the prosencephalon (forebrain), the mesencephalon (midbrain), and the rhombencephalon (hindbrain), which merges smoothly with the spinal portion of the neural tube. The prosencephalon develops into the cerebrum. The mesencephalon becomes the midbrain of the adult brainstem, and the rhombencephalon becomes the rest of the brainstem and the cerebellum (Table 2-1).
The three primary vesicles are not arranged in a straight line; rather, they are associated with two bends or flexures in the neural tube (Fig. 2-8A). One of these, the cervical flexure, occurs between the rhombencephalon and spinal cord but straightens out later in development. The second, the cephalic (or mesencephalic) flexure, occurs at the level of the future midbrain and persists in the adult as the bend between the axes of the brainstem and the cerebrum (see Fig. 3-1).
There Are Five Secondary Vesicles
As the brain continues to develop, two of the primary vesicles become subdivided. During the fifth week, five secondary vesicles can be distinguished (Fig. 2-9). The prosencephalon gives rise to the telencephalon (Greek for “end brain”) and the diencephalon (Greek for “in-between brain”); the mesencephalon remains undivided; the rhombencephalon gives rise to the metencephalon and the myelencephalon. The telencephalon becomes the cerebral hemispheres of the adult brain. The diencephalon gives rise to the thalamus (a large mass of gray matter interposed between the cerebral cortex and other structures), the hypothalamus (an autonomic control center), the retina, and several other structures. The metencephalon becomes the pons (part of the brainstem) and the cerebellum. The myelencephalon be-comes the medulla (the part of the brainstem that merges with the spinal cord).
In addition, a pontine flexure appears in the dorsal surface of the brainstem between the metencephalon and the myelencephalon (Fig. 2-9A). This flexure does not persist as a bend in the axis of the brainstem, but it does have important consequences for the configuration of the caudal brainstem. As the flexure develops, the walls of the neural tube spread apart to form a diamond-shaped cavity (hence the name rhombencephalon) so that only a thin membranous roof remains over what will become the fourth ventricle (Fig. 2-10). Thus the alar and basal plates, still separated by the sulcus limitans, come to lie in the floor of the fourth ventricle. The result is that in the corresponding part of the adult brainstem (rostral medulla and caudal pons), sensory nuclei are located lateral, rather than posterior, to motor nuclei. As discussed further inChapter 12, this is of some utility in making sense of the arrangement of cranial nerve nuclei.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree