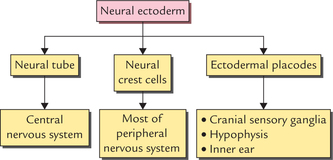
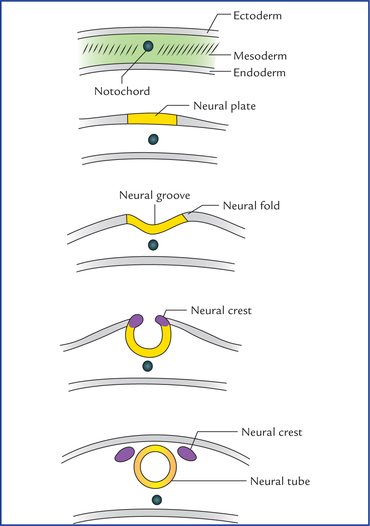
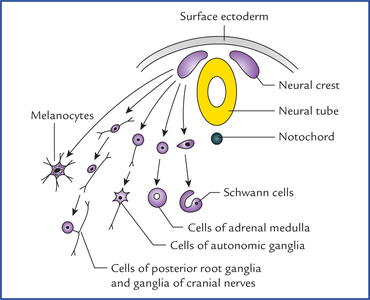
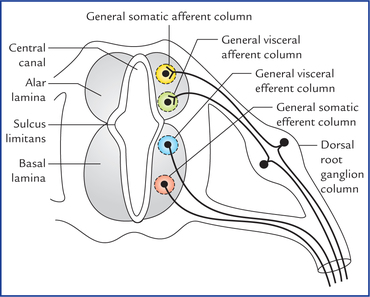
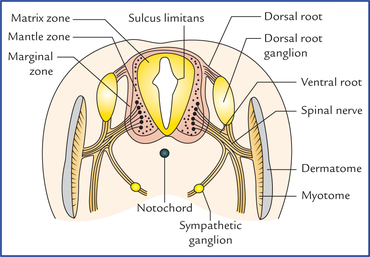
1 A study of development of the nervous system helps to understand its complex organization and the occurrence of various congenital anomalies. The specific cell population of the early ectoderm, which gives rise to entire nervous system and special sense organs is termed neural ectoderm. The neural ectoderm later differentiates into three structures: neural tube, neural crest cells, and ectodermal placodes. The neural tube gives rise to the central nervous system (CNS), the neural crest cells form nearly all the peripheral nervous system and ectoder-mal placodes contribute to the cranial sensory ganglia, hypophysis and inner ear (Flowchart 1.1). As the neural folds come together and fuse, the cells at the tips of neural folds break away from the neurectoderm to form neural crest cells. The surface ectoderm of one side becomes continuous with the surface ectoderm of the opposite side over the neural tube. The neural crest cells differentiate to form the cells of dorsal root ganglia, sensory ganglia of cranial nerves, autonomic ganglia, adrenal medulla, chromaffin tissue, melanocytes and Schwann cells (Fig. 1.2). The spinal cord develops from the caudal elongated part of the neural tube. The neural tube increases in thickness due to repeated mitosis of its epithelial lining. By the middle of 5th week of embryonic development, the transverse section of the recently closed neural tube (according to classical theory) reveals three distinct layers or zones. From within outwards these are: (a) matrix (ependymal) zone, (b) mantle zone, and (c) marginal zone (Fig. 1.3). Fig. 1.3 The differentiation of the neural tube into three distinct layers and zones and associated structures. Now according to current theory the wall of recently closed neural tube consists of only one cell type, the pluripotent neuroepithelial cells. These cells extend over the entire thickness of the wall and form thick pseudostratified neuroepithelium. The zonal appearance merely reflects the different phases of their proliferative cycle, the sequence being termed interkinetic migration. The cells of dorsal region or alar lamina are functionally afferent/sensory while those of basal lamina are efferent/ motor. The axons of cells of basal lamina leaving the cord as ventral roots join with the peripheral processes of dorsal root ganglia, to form the spinal nerves (Fig. 1.4). The two afferent columns of alar lamina receive axons from dorsal root ganglia. These are: 1. General somatic afferent column: It extends throughout the spinal cord and receives impulses from superficial (cutaneous) and deep (proprioceptive) receptors. 2. General visceral afferent column: It is confined to thora-columbar and sacral regions only and receives impulses from viscera and blood vessels
Development of the Nervous System
Formation of Neural Crest Cells
Development of Spinal Cord
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neupsy Key
Fastest Neupsy Insight Engine