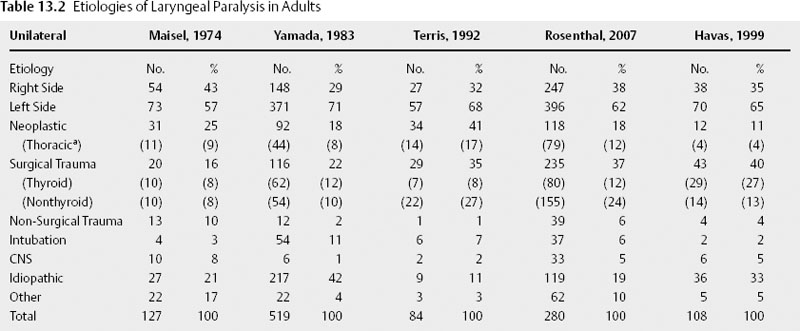
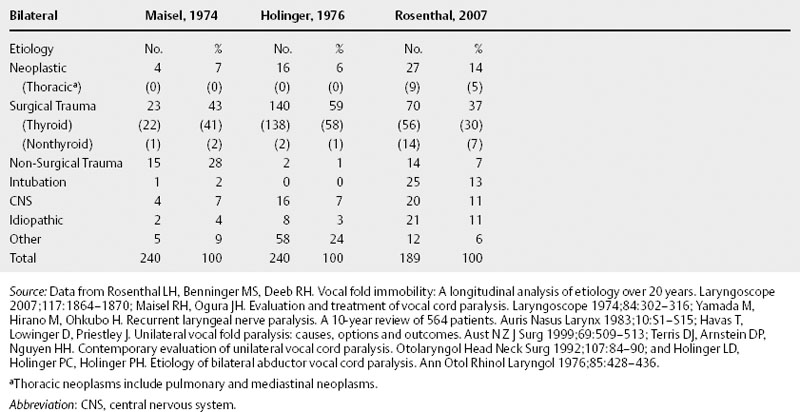
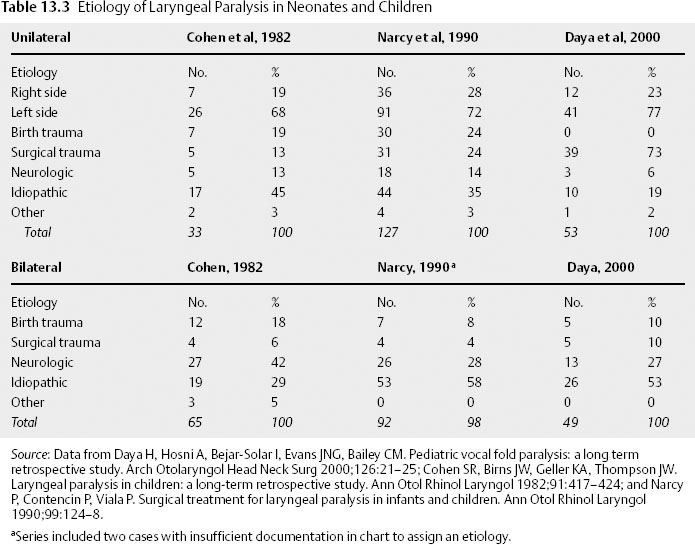
Chapter 13 In the 4th century B.C. Hippocrates recognized laryngeal paralysis as a cause of aspiration. In the 1860s, Gerhardt, with the help of indirect mirror laryngoscopy, first diagnosed the clinical condition of laryngeal paralysis.1 Twenty years later, Felix Semon2 observed a pattern of paramedian fold position in vocal paralysis and hypothesized that abductor nerve fibers were more susceptible to disease than adductor fibers. In the ensuing century otolaryngologists have observed multiple fold positions in laryngeal paralysis and gained a greater understanding of the complexities of the neuromuscular pathophysiology of this condition. The larynx provides the major functions of airway protection, airway support, and phonation. The nerve supply to the larynx is long and circuitous, highlighted by the left recurrent laryngeal nerve (RLN) coursing around the aortic arch. Complete interruption of laryngeal nerve pathway results in laryngeal paralysis. In the medical lexicon, laryngeal paralysis has evolved to erroneously represent the spectrum of partial to complete nerve deficit. It should be stressed that a true paralysis is a rare condition, and that most patients have a partial nerve deficit, or paresis. Complete paralysis of the larynx is usually seen with complete transection of the RLN and superior laryngeal nerve (SLN). More often an injury results in neuropraxic damage to a portion of the motor nerve. Other causes of partial deficits include an interrupted vascular supply and nerve regeneration resulting in laryngeal function months after the initial nerve injury. Therefore, most patients present with a paresis. The differential diagnosis and evaluation of vocal fold paralysis and paresis differ based on known or unknown etiology, bilateral versus unilateral disease, and whether the patient is a child or an adult. The etiologies of laryngeal paralysis and paresis are numerous (Table 13.1). Trauma (both surgical and nonsurgical), neoplasm, and central nervous system disease are the leading causes in both children and adults (Table 13.2 and Table 13.3). Often after an extensive evaluation a large number of cases remain idiopathic. Comparing unilateral and bilateral vocal fold paresis across age classification demonstrates that adults suffer unilateral paresis more often than bilateral disease.3–7 In contrast, unilateral and bilateral disease present with a similar prevalence in children.8–10 In both age groups, left-sided lesions account for around two thirds of unilateral cases, as the long anatomic course of the left RLN is more frequently interrupted during surgery and by pulmonary neoplasm.3,6–8,10–13 Across the past three decades the etiology of adult unilateral vocal fold immobility has remained stable. The primary causes are surgical trauma and extralaryngeal malignancy, with pulmonary and mediastinal tumor representing the most common locations. A review of the literature demonstrates that primary lung cancer is not distinguished from metastatic lung cancer.3,5–7,12–14 The next largest category is idiopathic, ranging from 11 to 42%.3,5–7,12,13 Central nervous system disorders, nonsurgical trauma, and intubation are common but less frequent causes of unilateral laryngeal nerve deficit. Iatrogenic injury is the leading cause of adult bilateral vocal fold paralysis. The singular operation responsible for the surgical trauma to both vocal folds is the total thyroidectomy. Fortunately, recent studies demonstrate a decreasing proportion of bilateral vocal fold paralysis due to thyroidectomy, decreasing from 58% to 30%.3 This decrease may be a function of subspecialization, as endocrine specialists are now primarily responsible for surgical management of thyroid disease. Intubation injuries commonly cause bilateral disease; however, it is rare for a malignancy to affect both RLNs. When malignancies result in bilateral disease, immobility occurs precipitously and is the result of a very aggressive neoplasm, such as an anaplastic thyroid tumor or submucosal tumor extension. In children and neonates, congenital central nervous system disorders are the leading cause of bilateral paralysis. Arnold-Chiari malformation type II is the most common central nervous system disease. Iatrogenic injury is a leading cause of unilateral vocal fold paralysis in children, with reports as high as 73%.8 Other causes such as cardiovascular anomalies and surgery are responsible for left vocal fold deficits. In neonates, birth trauma is a common cause of both bilateral and unilateral paralysis. As in adults, there is a high percentage of unilateral idiopathic laryngeal paresis. On the other hand, bilateral idiopathic paresis, which is rare in adults, is quite common in children. Viral illness is speculated to cause a large percentage of idiopathic vocal fold paresis. There are many case reports of laryngeal paresis associated with reactivated herpes simplex virus (HSV) and more rarely cytomegalovirus, Epstein-Barr virus, and postpolio patients.15–19 In a 2001 series Koufman et al20 presented separate idiopathic and viral etiologies, with the viral cause defined by the patient’s report of recent viral upper respiratory infection. This study demonstrated around 40% of previously defined idiopathic cases to be of viral etiology. A series of SLN paresis reported by Dursun et al21 found a viral etiology in 94% of patients.21 Although case reports have demonstrated HSV-positive serology, a large-scale study has not been performed.18–21 If a large series correlates HSV-positive herpes serology in patients with idiopathic vocal fold paresis, antiviral therapy could be implemented as treatment, as is the current practice in subclinical viral labyrinthitis. Two rare causes of laryngeal paralysis are familial disease and vincristine chemotherapy. The hereditary forms cause bilateral paralysis and include patterns suggesting both an X-linked and autosomal dominant inheritance.22–24 No gene has been identified at this time. Vincristine’s peripheral neurotoxicity has been reported to cause both bilateral and unilateral laryngeal paralysis in children and adults.25,26 The paralysis usually resolves with discontinuation of chemotherapy.25,26 Neural dysfunction of the larynx can adversely affect voice, ventilation, and airway protection. The resulting clinical manifestations are variable and dependent on the degree of paresis. With regard to voice, dysphonia and vocal fatigue are common. Vocal fatigue is a result of exhausted compensating musculature. A neonate may be unable to cry due to the large glottic gap. Adult patients with unilateral vocal fold immobility frequently present with a weak and breathy voice. In SLN deficit the voice often displays a loss of projection and power, especially noticeable at a high pitch–a result of decreased tension on the affected fold. Koufman et al4 demonstrated that voice change is present in 100% and vocal fatigue in 76% in a review of 50 patients presenting with paresis. Diplophonia (40%), or two tones produced by different tension of the vocal folds, and pain with phonation (10%) were found to be less frequent. Exertional phonation in combination with dysphonia, vocal fatigue, and odynophonia is a sign of excessive supraglottic activity.4,27 This condition is frequently due to abnormal muscle tension in the larynx. Its presence should alert the physician to focus the examination on the glottis, which often reveals incomplete closure. Muscle tension dysphonia usually represents a compensatory behavior for a laryngeal deficit. Although it is important to exclude a nerve injury, the vocal fold weakness may be due to a movement disorder, such as Parkinson disease, a soft tissue deficit or mass, or trauma, as in the case of arytenoid dislocation. Stridor is often seen in bilateral vocal fold paralysis and causes dyspnea and respiratory distress in a small percentage of adult patients. This is a result of a fixed midline fold position, which preserves voice function and usually prevents aspiration. In children, stridor is the leading symptom and is frequently seen in unilateral disease as well as in bilateral paralysis. Cohen et al10 reported stridor in 71% and obstruction in 44% of children with laryngeal paralysis. Dysphonia (35%), dysphagia (38%), and aspiration (14%) were encountered less often. Airway distress, cyanosis, and apnea are common in bilateral immobility, though some infants may remain asymptomatic for up to 6 months; however, when their oxygen needs increase, they become symptomatic. Aspiration may be seen in unilateral vagal or RLN paralysis because the affected fold is often in the paramedian position and cannot fully close. These patients have difficulty protecting the airway and lack a sufficient cough that results in aspiration. Dysphagia is less severe with an RLN injury alone than is a high vagal injury that includes sensory denervation. In newborns, aspiration may manifest as recurrent pneumonia. SLN deficit rarely causes choking and aspiration; when present, these symptoms are a function of sensory deficit from the external nerve branch. Aspiration almost always indicates bilateral SLN denervation, as unilateral sensory deficit is usually compensated by the contralateral sensation.21,28 The four nerve deficits that can cause vocal fold paralysis are central nervous system disorder, high vagal lesion, RLN injury, and SLN injury. Each lesion has a classic pattern of muscle weakness and vocal fold position. Clinically, patients present with variable vocal fold position that is a function of the degree of paresis, the presence of synkinesis, mechanical trauma, and the patient’s laryngeal anatomy, which substantially differs between men and women. Although every patient with a specific nerve deficit does not present with the same fold position, these common patterns, if observed, may assist in the diagnosis and evaluation. Central nervous system injury causing laryngeal paresis usually indicates brainstem dysfunction. Brainstem lesions frequently occur as a result of a stroke or tumor and are associated with involvement of other cranial nerves. The lesion may only affect motor innervation due to injury to the dorsal and ventral nucleus ambiguus. Therefore, the presentation includes motor loss of the pharynx in addition to laryngeal deficit.29 A distinguishing feature of central nervous system deficit is the lack of a sensory or secretory deficit.30 As is commonly observed with upper motor neuron deficit, the initial flaccid paresis segues into a spastic paresis with slow laryngeal movement and a decreased range of abductor and adductor function.31 A shift in the posterior glottis to the normal side with the onset of speech may also be seen.32 Cortical lesions tend to spare the vocal folds due to diffuse interhemispheric connections that provide bilateral input to laryngeal nerve pathways. The unique combination of motor, sensory, and secretory deficit usually indicates extracranial vagal injury.30 This is a result of the combined injury to the recurrent and superior laryngeal nerves pathways within the vagus. In high vagal paralysis, the fold is frequently paramedian, with bowing and atrophic changes resulting in a glottic gap. The two folds are at different levels and in an asymmetric position.32 In unilateral disease, the healthy fold might cross midline; however, most patients suffer mild to moderate hoarseness due to incomplete glottic closure and vibratory asymmetry. Nerve injury affects the muscles unequally, and the loss of opposing forces results in the vocal process falling anteromedially. More frequently, nerve injury affects the muscles unequally. The loss of opposing forces results in the vocal process falling anteromedially. An isolated RLN injury is similar to a high vagal lesion with the affected fold(s) in a paramedian position. On the other hand, there is no sensory or secretory deficit and the cricothyroid muscle remains fully innervated. Denervation of the posterior cricoarytenoid alone results in abduction deficit, whereas isolated thyroarytenoid deficit causes atrophy of the affected fold. This paretic condition leads to premature and greater lateralization with an abnormal mucosal wave.32 As with vagal injury, bilateral deficit does not usually provide enough airway support and necessitates urgent tracheostomy. An SLN deficit results in ipsilateral cricothyroid muscle weakness. Diplophonia is commonly encountered due to the different vibratory frequency of the affected nerve from the healthy side. The most consistent signs are a shortened bowed vocal fold and sluggish motion.21 Debate remains on whether a cricothyroid deficit alone can cause a deviation of the larynx toward the affected nerve.33 An algorithm can be used for the evaluation of laryngeal paralysis (Fig. 13.1). Its purpose is to provide a comprehensive and financially efficient decision tree of diagnostic studies. An extensive evaluation searching for the cause of idiopathic immobility can be expensive, yet often it is clinical suspicion combined with a directed workup that reveals a diagnosis. In a study on the evaluation of unilateral vocal fold paralysis, Terris et al13 reported that otolaryngologists with greater experience pursue a briefer and less expensive evaluation for unilateral vocal fold paralysis. The first issue the clinician must address is airway stability. After determining or obtaining airway stability, a useful question to ask the patient is, “Did something happen that caused the problem with your larynx?” A majority of patients present with a known cause of paralysis; studies of unilateral paralysis show that number to be between 57 and 63%.5,13
Diagnosis and Evaluation of Laryngeal Paralysis and Paresis
Etiology
Signs and Symptoms
Neuropathology
Evaluation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree