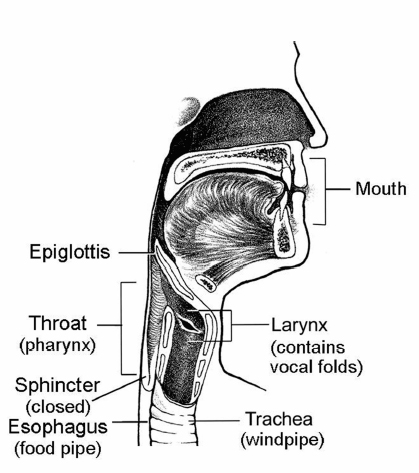
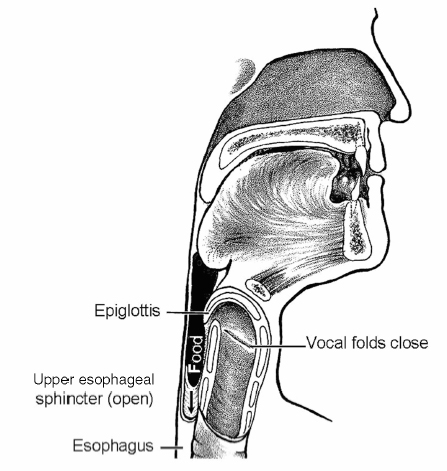
Chapter 16 Swallowing is a highly complex balancing act that most people do not think about until a bit of food or a pill is caught in the throat or until more than an occasional sip of liquid is aspirated. Approximately 30 oral and pharyngeal muscles and multiple nerves must perform precisely on cue so that the upper aerodigestive tract is reconfigured from a mechanism that channels air for breathing and speaking (Fig. 16.1) to a food-propelling mechanism that safely accomplishes ingestion (Fig. 16.2). Fig. 16.1 Aerodigestive tract channeling air for breathing from the nose and mouth through the open larynx into the lungs and back up and out. For speaking, air is channeled similarly, but the vocal folds vibrate to produce voice.(From Weihofen D, Robbins J, Sullivan P. The Easy to Swallow, Easy to Chew Cookbook. New York: John Wiley; 2002. Adapted with permission.) Fig. 16.2 Aerodigestive tract reconfigured from an air channel to a food-propelling mechanism. The tongue propels food into the throat; the epiglottis covers the larynx, which is the airway entrance; and the vocal folds close to protect the trachea and lungs from foreign material. (From Weihofen D, Robbins J, Sullivan P. The Easy to Swallow, Easy to Chew Cookbook. New York: John Wiley; 2002. Adapted with permission.) The four morphologic regions serving these purposes are the oral cavity, pharynx, larynx, and esophagus. Of these, the first three collectively are termed the upper aerodigestive tract because they also serve the airway-dependent functions of respiration and speech production. In humans, with our upright posture, it is the adjacent position of the anatomy for breathing to the anatomy for food passage that unfortunately facilitates gravitational influences on food to flow into an unprotected airway. The larynx is essential to safe swallowing and is neurologically wired to protect the airway from entry of food, liquid, medications, or secretions. However, a perspective of dysphagia that focuses on the presence or absence of aspiration, as it relates only to the larynx, ignoring the critical elements of the propulsive pump for bolus transfer and the opening of the upper esophageal sphincter (UES) for clearance, would result in limited and largely erroneous treatment plans that could prove fatal to the patient who aspirates in the presence of an intact larynx. Indeed, essential to normal swallow physiology, including three levels of airway protection, is normal clearance of food from the oropharynx so that when the larynx returns to an open inspiratory position, no residual foreign material is in oropharyngeal recesses to be inhaled and aspirated. Further, esophageal integrity is critical as well to minimize aspiration that may occur long after swallowing is completed due to material maintained in the esophagus (intraesophageal stasis and reflux)1 or below, in the form of gastroesophageal reflux. These latter events may occur in the presence of an intact larynx. Thus aspiration may occur at mealtime or at other times. When aspiration is during the meal, particularly early in the meal, then the biomechanics and anatomy of the oropharyngeal swallow or the esophagus must be carefully considered. If aspiration is reported to occur after the meal, and often seemingly not present during eating, then intraesophageal or gastroesophageal reflux is more likely the culprit and warrants evaluation. In the latter scenario, the larynx is often functionally intact but reacting to the refluxate that moves posteriorly/superiorly and may enter the airway, damaging the larynx and causing related sequelae. Disease or damage to any region along the neural axis from the cerebral cortex to muscles of the oral cavity, pharynx, larynx, and esophagus may produce dysphagia ( Table 16.1). The temporal relationship of dysphagia to neurologic disease is variable, with some disorders associated with the acute onset of dysphagia (e.g., stroke) and other, typically degenerative disorders (e.g., Parkinson disease [PD]) associated with a gradual onset of swallowing problems (Table 16.2). Stroke has been the most studied of the neurologic etiologies of dysphagia. Since the advent of neuroimaging techniques, it is well established that in addition to brainstem or bilateral hemispheric stroke, a single stroke involving either cerebral hemisphere may produce significant and protracted dysphagia.2,3
Diagnostic-Based Treatment Approaches
Neurologic Conditions Affecting Swallowing
| Medical |
| Botulinum toxin injection |
| Dilation |
| Nasogastric tube |
| Surgical |
| Cricopharyngeal myotomy |
| Laryngeal framework surgery with or without arytenoid adduction |
| Vocal fold augmentation—injection of medialization thyroplasty |
| Tracheostomy |
| Laryngotracheal separation |
| Hypopharyngoplasty |
| Stents |
| Cervical esophagostomy, gastrostomy, jejunostomy |
| Central nervous system |
| Nondegenerative |
| Stroke |
| Traumatic brain injury |
| Cerebral palsy |
| Neoplasms |
| Neck surgery |
| Carotid endartectomy |
| Cervical fusion |
| Degenerative |
| Dementia |
| Movement disorders |
| Parkinson disease |
| Progressive supranuclear palsy |
| Olivopontocerebellar atrophy |
| Huntington’s disease |
| Wilson’s disease |
| Multiple sclerosis |
| Peripheral nervous system |
| Amyotrophic lateral sclerosis |
| Postpolio syndrome |
| Myasthenia gravis |
| Guillain-Barré |
| Myopathy |
| Myotonic dystrophy |
| Oculopharyngeal muscular dystrophy |
| Inclusion body myositis |
| Polymyositis |
| Dermatomyositis |
| Hyper- and hypothroidism |
Neurologic disorders may affect the oropharyngeal or esophageal phases of swallowing. Although patterns of swallowing kinematics emerge from underlying etiologies, none has been shown with adequate statistical power to be identified as providing distinguishing hallmarks in swallowing characteristics that differentiate one neurologic disease from another.
Symptoms of neurogenic dysphagia may be obvious, characterized by coughing with swallowing or a patient’s complaint of pharyngeal retention or a feeling of something “stuck.” However, unlike dysphagia resulting from head and neck cancer in which the dysphagia symptoms are apparent, swallowing problems resulting from neurogenic disorders may not be as obvious because the disorder may more commonly affect the sensory system.4 Thus, a cough reflex may not be evoked with aspiration, or a patient may not be aware of pharyngeal residue. Moreover, neurologic disorders may affect a person’s cognition or language, which can limit a patient’s awareness of, or ability to communicate about, a swallowing disturbance.
Diagnosis and Treatment Evaluation
Evaluating the Swallow
Before determining the type of treatment to employ for neurogenic dysphagia, the specific biomechanic or physiologic disorder(s) of the dysphagia (e.g., decreased anterior laryngeal movement, delayed evocation of the pharyngeal swallow response) must be identified. To identify the exact nature of the swallowing disorder, an instrumental swallowing examination must be completed. The videofluoroscopic swallow study (VSS) is generally the method of choice in evaluating oropharyngeal dysphagia in that kinematic events comprising all stages of swallowing, from the oral cavity through the esophagus, can be visualized and examined while in motion, and the dynamics of bolus flow also are visualized.
Evaluating the Treatment
By employing an instrumental examination to determine the exact mechanism of dysphagia, one can use a specifically designed treatment to target the underlying biomechanical problem during the fluoroscopic examination. Certain compensatory strategies such as postures and food or fluid consistency modification as well as swallowing maneuvers should be attempted during the instrumental examination to determine their effectiveness. Other less immediate treatments, such as rehabilitative, medical, surgical, or prosthetic, should be employed to target the area of dysfunction identified on the instrumental examination.
Treatment
Medical Treatment
As with direct swallowing rehabilitation, any medical or surgical intervention for swallowing should be performed to address the pathophysiology underlying specific swallowing biomechanical contributors to the dysphagia identified on the instrumental examination. No randomized, controlled clinical trials have been conducted to determine the effectiveness of medical or surgical interventions on the rehabilitation of neurogenic dysphagia.
Botulinum toxin (Botox) type A has been used in treating UES dysfunction. Studies generally report improved swallowing on the instrumental examination or by patient report,5–10 with results lasting from 1 to 14 months post-injection.7 However, these studies are limited in that they are retrospective, and completed in heterogeneous populations; no study has incorporated a placebo control.
Botox type A injection into the salivary glands (parotid, submandibular) also has been used to treat sialorrhea (hypersalivation) in neurogenic patients including those with PD and amyotrophic lateral sclerosis.11,12 Double-blind, placebo-controlled studies have been completed in patients with PD using Botox type A13 and type B.14 Subjective improvement in drooling was identified in the patients receiving either type of Botox, whereas the placebo group in both studies reported no change.
Dilation, either pneumatic or bougienage, may be used to treat cricopharyngeal dysfunction; however, no study has specifically focused on this procedure in a group of patients with neurogenic dysphagia. Symptomatic response to cricopharyngeal disruption with either dilation or myotomy was studied in a heterogeneous group of subjects.15 Results revealed 58% of subjects who underwent dilation had a subjective improvement in swallowing.
Surgical Treatment
Patients with severe swallowing disorders may require surgical intervention at some point in the course of their illness. Surgery in the patient with a swallowing disorder aims to (1) improve passage of the bolus, (2) prevent aspiration, or (3) facilitate non-oral feedings. Surgery is indicated in patients with neurogenic dysphagia when (1) there is clearly a surgical lesion, such as a tumor, stricture, or diverticulum, which is generally a coincidental finding with this population; (2) a deficit, such as a vocal cord paralysis or UES dysfunction, persists and is symptomatic despite a period of observation and appropriate nonsurgical management; (3) there are actual or imminent complications from chronic aspiration despite the cessation of oral feedings; (4) remaining without oral feedings to prevent aspiration is an unacceptable alternative to the patient; or (5) there is a need to establish a route for long-term non-oral feedings.16,17
Treatment of Laryngeal Incompetence
Laryngeal incompetence can result from vocal cord paralysis, a sensory deficit, or mechanical disruption and scarring. The most critical element of the sphincteric function of the larynx preventing aspiration is the closure of the true vocal cords during deglutition. A vocal cord paralysis can cause aspiration by producing incomplete glottic closure during swallowing, and an ineffective cough. Loss of laryngeal sphincteric function also decreases subglottic pressure during swallowing, which is felt to decrease efficiency of swallowing and increase aspiration.18 Aspiration is more commonly seen when laryngeal paralysis causes a deficit in closure at the posterior aspect of the glottis.
The techniques devised primarily to improve phonation in patients with vocal cord paralysis also may aid in the management of aspiration. The details of these procedures are discussed elsewhere in this text, but essentially include laryngeal framework surgery with or without arytenoid adduction, and injection thyroplasty. Aspiration can be corrected by medialization of the immobile vocal cord, thus permitting the contralateral vocal cord to achieve glottic closure when it is adducted. Framework surgery in which medialization is achieved by inserting an implant lateral to the vocal cord (type I thyroplasty) has been demonstrated to decrease aspiration in a variety of disorders associated with vocal cord paralysis and dysphagia, and to facilitate elimination of the need for tracheostomy.19 The addition of an arytenoid adduction procedure is necessary when the laryngeal deficit includes a significant posterior glottic gap. Despite positioning thyroplasty implants to extend to the level of the vocal process of the arytenoids, the arytenoid adduction procedure is more effective in closing posterior gaps. The procedure is performed by inserting a suture into the muscular process of the arytenoid. By applying anterior traction and fixing the suture to the lateral ala of the thyroid cartilage, the arytenoid is rotated, thus positioning the vocal process in a median position. The arytenoid adduction procedure is usually performed in combination with a thyroplasty medialization procedure, although it can be performed as the initial step, after which the need for thyroplasty is assessed.20–22
Medialization thyroplasty and arytenoid adduction procedures may be performed under general anesthesia, but they are usually performed under local anesthesia to allow intraoperative monitoring of the voice, airway, and adequacy of glottic closure. Thyroplasty procedures are reversible (i.e., the implant can be easily removed) and, therefore, can be performed even if return of function may occur at a future time. On the other hand, because of the dissection at the cricoarytenoid joint, arytenoid adduction should be considered a permanent alteration of laryngeal anatomy, and should be performed only when no return of function is anticipated (e.g., after transection of the vagus nerve during skull base surgery).
Injection thyroplasty accomplishes medialization of the immobile cord by injecting the cord directly to close an identified glottic gap. Many materials are currently used for injection including fat, fascia, gelatin powder, collagen, and acellular micronized human dermis.23 The advantage of injection thyroplasty is that the procedure is less invasive and can sometimes be performed percutaneously as an office procedure. Also, resorbable material such as gelatin powder can be used for short-term trials. These injection procedures, however, do not afford the control and precision that surgical thyroplasty and arytenoids adduction provide. Resorption of injected materials can also make long-term results unpredictable. Injections can be used as an adjunct to surgical medialization.24,25
A recently introduced hypopharyngoplasty procedure may be a useful adjunct in the management of patients with laryngeal incompetence and pharyngeal paralysis.26 The procedure is performed at the time of thyroplasty and arytenoid adduction. Insensate and redundant piriform sinus mucosa on the paralyzed side is resected to prevent dilation and pooling, and the inferior constrictor muscle is advanced anteriorly to increase tone. To date, the procedure has been performed only by its progenitors on eight patients who were reported to have good outcomes.
When glottic incompetence results from mechanical disruption or scarring (e.g., after trauma or partial laryngeal resection), one may not be able to improve competency with standard implants or injections. Procedures for specific situations need to be employed, such as the subperichondrial implantation of a portion of the superior thyroid cartilage to medialize the vocal cord in patients after laser treatment of glottic carcinoma.27 Partial collapse of the cricoid cartilage has also been used to correct glottic incompetence after extended supraglottic laryngectomy.28
The appropriate application of procedures for laryngeal incompetence is dependent on a complete multidisciplinary evaluation of the causes of aspiration in an individual patient. The position of an immobile vocal cord, the status of posterior glottic closure, associated dysmotility of the tongue and pharynx, and general strength and mental status contribute to the likelihood of aspiration. The procedures discussed above are effective for localized, well-defined areas of laryngeal incompetence. The effectiveness of these procedures is diminished in situations where there is associated diffuse incoordination and sensory loss, as may occur in stroke patients or patients with advanced neuromuscular disorders.
Treatment of Cricopharyngeal Muscle Dysfunction
Failure of the cricopharyngeus muscle, the major component of the UES, to relax appropriately may cause dysphagia, pooling of secretions, and aspiration. There is much conflicting information in the literature regarding the incidence and management of cricopharyngeal dysfunction (spasm or incoordination during the pharyngeal phase of swallowing).29,30 Evidence supporting the use of cricopharyngeal myotomy for neurogenic dysphagia is limited. No trial for cricopharyngeal myotomy for neurogenic dysphagia has been conducted. If one were performed, it would have to include those with a hyperactive cricopharyngeus muscle, and exclude the patients with more generalized dysfunction. Such a problem was noted in the study reviewing cricopharyngeal myotomy for dysphagia in head and neck cancer patients. In this study by Jacobs et al,31 no significant differences were seen in swallowing between patients who had a myotomy and those who did not. Of course, the entire cohort included patients with multiple swallowing disabilities, which are not likely to be improved with myotomy alone. The studies to date for myotomy in the neurogenic dysphagia population have used subjective, rather than objective, measures, and had small sample sizes.32–34 Dysfunction associated with a systemic disorder (e.g., parkinsonism, myasthenia gravis, or polymyositis) may improve with specific treatment of the disorder or specific swallowing rehabilitation. Persistent cricopharyngeal dysfunction may need to be addressed directly; however, before this can be undertaken, the exact nature of the disorder must be understood, as isolated cricopharyngeal dysfunction is rare in patients with neurogenic dysphagia. In this population, decreased anterior hyolaryngeal traction to open the UES is the etiology often related to reduced lingual pressure generation and therefore reduced intrabolus pressures to assist in optimizing UES opening, not failure of the muscle to relax35. In these cases, myotomy would not improve swallowing.
Cricopharyngeal myotomy is the most effective treatment for persistent, isolated cricopharyngeal dysfunction.36,37 This has been shown to be effective in some patients who suffered lateral medullary brainstem stroke in which the relaxation of the UES is disinhibited. The procedure is most successful when combined with a behavioral opening maneuver such as the Mendelsohn,38 because even after the surgical “relaxation” of the UES is accomplished, opening of the sphincter must occur and that is accomplished best with a voluntary mechanical maneuver. Lateral medullary stroke patients (particularly the unilateral patients who are the ones most likely to survive) are generally very cognitively intact. The operation can be performed endoscopically, usually using a CO2 laser, but is still most often performed via an external approach. In the endoscopic approach, the muscle is transected submucosally in the midline posteriorly to the prevertebral fatty tissue.39
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







