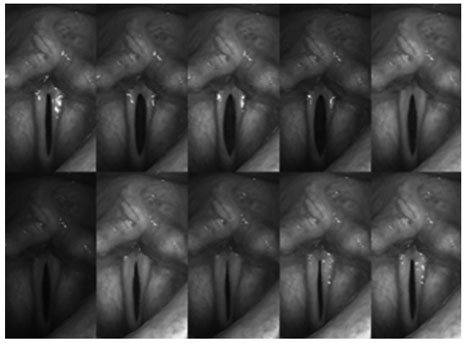
Chapter 14 Vocal fold incompetence is a pathologic state characterized by the reduction of efficient vocal fold vibration in the setting of normal aerodynamic support. It can arise from reduced vocal fold adduction, loss of vocal fold volume, alteration of vocal fold viscoelasticity, or from a combination of the three. In all cases the entrained oscillation of the vocal folds is hindered, and increased subglottal pressures and airflow are required to power glottic sound production. Patients complain of vocal fatigue, reduced vocal projection, and a breathy voice quality.1 Reduction of glottic closure is commonly seen in unilateral vocal fold paralysis.2,3 Common causes of this paralysis include iatrogenic injury to the recurrent laryngeal nerve (RLN), viral palsy, and neural compression from intrathoracic processes.4 The denervation of the intrinsic laryngeal musculature prevents full closure of the glottis during phonation and also introduces viscoelastic asymmetry from the flaccidity of the affected vocal fold.5 In these cases, injection of filler into the vocal fold can address glottal competence by moving the affected vocal fold medially.2,3,6–8 However the position of the arytenoid and the issue of viscoelastic asymmetry are difficult to address precisely. Partial immobility of the vocal folds may also result from synkinetic reinnervation of the denervated cricoarytenoid unit, paresis from a partial RLN injury, or paresis from a superior laryngeal nerve (SLN) palsy.9–11 These conditions produce glottal incompetence with lesser degrees of severity. Given the smaller contribution of arytenoid misplacement, injection techniques may have a more beneficial effect. Vocal fold immobility may also result from fixation of the cricoarytenoid (CA) joint. Most commonly seen after prolonged intubation and described well by Bastian and Richardson,12 periarticular fibrosis of the CA joint reduces the abduction and adduction of both CA units, leaving a large gap between both the arytenoid and the musculomembranous segments. Fixed arytenoids can also result from end-stage rheumatoid arthritis, osteoarthritis, or gout within the CA joint, creating an identical dilemma.13–18 Injection techniques may be of benefit in medializing the anterior (musculomembranous) segment, but they neither restore mobility to the CA joints nor provide closure to the posterior (cartilaginous) segment. Viscoelastic alteration of the vocal folds is generally not an important contributor to glottal incompetence because innervation is intact and tension is maintained. Neuromuscular discoordination as seen in spasmodic dysphonia and Parkinson disease can also produce glottal incompetence, not from an inability to achieve glottic closure or a change in viscoelasticity but rather from involuntary motion of the CA units. Glottic closure is not well synchronized with respiration, producing untimely exit of air through the glottis and a loss of entrained oscillation. Although neuromuscular discoordination has historically been managed at least in part by open laryngoplastic techniques, injection techniques may be aimed at augmenting the musculomembranous segments to reduce the size of the glottic gap at times of involuntary vocal fold separation, thereby reducing the degree of air loss.19–21 Other causes of glottic incompetence are related to actual loss of vocal fold tissue. Often referred to as presbylaryngis, aging changes to the vocal folds produce a loss of volume in the vocal fold cover (lamina propria [LP]).22,23 The glottic appearance on office endoscopy is that of “bowing,” in which a spindle-shaped aperture is described. Despite the adequate adduction of the arytenoids, air loss through this incompetent anterior valve is inevitable. Injection of the musculomembranous segment can produce improved closure by approximation of the oscillating surface and has been used also to replace some of the lamina propria lost in the aging process.24 Increasing efforts are being made to reconstitute the lamina propria using fillers of appropriate viscoelasticity, either directly introduced or through means of stimulation, such as growth factors and tissue engineering.25–27 These techniques likely represent the future of vocal fold restitution and are discussed later in the chapter. Vocal fold tissue can also be lost in extirpative procedures related to cancer or infiltrative processes such as amyloidosis or sarcoidosis.28–30 These resections, performed either with cold instruments or with CO2 laser leave fixed focal gaps in the anterior segment of the glottis, creating a constantly inefficient phonatory mechanism. Attempts at closure of these gaps have targeted the medialization of the concave area with injection techniques and open laryngoplasty.31–33 Restitution of the layered microstructure of the hemilarynx has been performed in animal models using scaffolds of sheeting that allow for influx of regionally appropriate tissue.34 Repair of macro- and microdefects will certainly be enhanced by using tissue engineering as techniques advance to human trials. Of note, tissue defects from extirpative procedures fill in partially during the healing phase, thereby closing some of the initial defect. However, the native pliable tissue is replaced by scar that has reduced pliability relative to the original tissue. Therefore, even in cases of complete “fill-in” the patient is doomed to have some degree of glottal incompetence due to the side-to-side viscoelastic difference. The last major contributor to vocal fold incompetence is pure loss of pliability within the lamina propria. This change can result from iatrogenesis, developmental anomalies, and chronic basement membrane zone injury from phonotrauma.35 Iatrogenic reduction of vocal fold pliability is likely seen most often after repeated treatment of recurrent respiratory papillomatosis.36 Application of the CO2 laser has beneficial effects with respect to hemostatic reduction of massive papillomata.37 However, the surrounding injury zone induces repair with scar, which has reduced viscoelastic qualities relative to native LP. Tissue biopsies that create a defect also induce tissue fill-in with scar, reducing the local pliability. Developmental anomalies such as sulcus vocalis types 2 and 3 produce local loss of pliability by replacing normal tissue with epithelium and scar and by interrupting the mucosal wave as it propagates from the inferior to superior lips of the vocal fold.38 Treatment strategies have been directed at restoration of a normal vocal fold microstructure and replacement of the dead space with a pliable implant such as alloderm.39 Injection techniques can introduce fillers but cannot separate the invagination of the vocal fold from the deep ligament. Phonotraumatic lesions of the vocal folds are very common and represent a spectrum of severity that is guided by how much scar has been deposited at the basement membrane zone. Nodules, for example, have been shown histopathologically to represent alteration of the normal microarchitecture with deposition of fibronectin and disruption of the smooth basement membrane.35 Patients with vocal fold incompetence have similar clinical presentations and endoscopic findings. Glottal inefficiency requires increased effort and airflow for voicing. As patients fatigue from speaking, their ability to project is also diminished. Particularly in cases of reduced pliability, the upper singing range may be eliminated.32 Additional comorbidities (advanced age, malignancy, neurologic weakness) may also contribute to a lack of energy sufficient for respiratory support. On physical examination, patients with vocal fold incompetence have a reduced maximum phonation time, a breathy voice quality, and possibly compensatory pitch elevation or supraglottic compression. A basic mirror examination proves useful in determining the pathology of gross vocal fold motion and visible mass lesions, but does not sufficiently evaluate pliability changes. Rigid telescopic strobovideolaryngoscopic exam is still the gold standard for evaluation of viscoelastic qualities of the vocal folds and for minor anatomic detail of the epithelium and LP.40 Digital archiving of the examination allows slow-motion review of gross adduction and abduction and helps reveal the glottal gap uniformly evident in this disorder. For example, in cases of vocal fold atrophy with aging, a spindle-shaped aperture is obvious (Fig. 14.1), particularly when compared with a normal subject. It is important to note if there is glottal inefficiency even in the modal range because the vocal folds are less taut and the LP has a greater ability to close. Unilateral defects are also visible in cases of prior biopsy and scarring montage. Here the normal vocal fold propagates to midline but the abnormal stiffness of the affected side reduces adequate entrainment. It is not unusual to have full visualization of the glottis reduced because of compensatory supraglottic compression. Perhaps the major appeal of vocal fold injection augmentation is the relative ease of the procedure and the likelihood of immediate symptom relief. Essentially, the procedure involves injection of a substance into the vocal fold to passively displace the leading edge of the affected vocal fold toward the midline. The major goal of injecting filler substances in the vocal fold is achievement of glottic competence with improvement of voice and improved airway protection during swallowing. It is unrealistic to expect most patients to achieve a completely normal voice. Usually the immediate response to restoration of glottic competence is that the patient describes less effortful phonation and production of a louder voice. Perceptually, an improved frequency and intensity range can be appreciated. Injecting a filler substance into the vocal fold is just one of several useful approaches to correcting glottal incompetence. It does not necessarily resolve intrinsic pathologic tissue characteristics, such as scarring or muscle atrophy, nor does it correct neuromuscular alterations like Parkinson disease that might cause glottic insufficiency. In unilateral paralysis, successful injection results in glottic closure occurring during active adduction of the contralateral vocal fold. Using only topical anesthesia, injection augmentation can be easily and quickly performed in an office setting, and it is usually well tolerated by patients. In cases of routine unilateral vocal fold paralysis, augmentation injection is best accomplished by sculpting the affected side to resemble the contour of the contralateral fold. There are several key points essential for achieving optimal results: Fig. 14.1 Montage of telescopic rigid strobovideolaryngoscopic images from a patient with bilateral vocal fold atrophy. Note that the vocal folds never achieve complete closure. Also note the longitudinal spindle-shaped glottic aperture characteristic of vocal fold atrophy seen during sustained phonatory tasks. Although there are many substances to choose from today, such was not always the case. The evolution of injection laryngoplasty began with a simple goal of filling space with an inert substance that could be easily injected to achieve glottic competence. Next we tried bioimplants because they more closely resembled tissues in the vocal fold lamina propria. Rather than posing a risk of granuloma or migration, the bioimplants (collagen, fat, and fascia) tended to be assimilated and replaced with host tissue. Bovine and human-derived materials were introduced including xenographs, homographs, and autogenous material. Historically, in 1911 Brunings injected paraffin via indirect laryngoscopy to medialize an immobile vocal fold. Paraffin allowed for adequate medialization with improved voice but often led to troublesome paraffinomas. Eventually, it was replaced by other inorganic alloplastic materials including silicone, Teflon,41,42 Bioplastique (a less viscous form), and, more recently, suspended hydroxylapatite Radiesse (Bioform Medical, San Mateo, CA).43,44 Due to foreign-body reaction granulomas and fibrosis caused by the original alloplasts,45–49 alternatives were sought. Bioimplants are histologically better tolerated, and typically they in large part become incorporated in the host tissues. The goal in injecting vocal folds with bioimplants is to place a substance that has potential to replace normal lamina propria cells and extracellular matrix. Bioimplants also induce host tissue in growth, avoiding foreign-body granulomatous reactions and favoring restoration of favorable viscoelastic properties. Biologic implants induce this tissue in growth by providing a matrix that facilitates revascularization and in growth of active fibrocytes.50 In assessing the effect of bioimplants, persistence of the correction of vocal fold medialization and function is more relevant than actual persistence of the graft material. Bovine collagen (Zyderm, Collagen Corp., Palo Alto, CA) was the first bioimplant tried51 ; the cross-linked form (Zyplast, Collagen Corp.) offered decreased risk of allergic reaction but proved less suitable for very superficial use. Both materials appeared to soften scar tissue and were shown to be suitable for small glottic gaps, focal scars, and tissue defects.24 Autologous collagen (Vocalogen) proved effective and safe,52 but it required a separate procedure to procure the tissues and long waits for processing the material, and it was costly. It is no longer available. Subsequent bioimplants employed included autogenous fat7,25,53 and fascia,54 freeze-dried irradiated human fascia (Fascian, Fascia Biosystems, Beverly Hills, CA), and micronized homologous dermis (Cymetra, Life-Cell Corp., The Woodlands, TX).8 Concerns of allergic reaction initially limited the use of bovine collagen, but some practitioners still find it useful as a temporary filler and for very superficial applications. Injected fat is difficult to contour in the vocal fold, tends to backflow at the injection site, and requires gross overinjection of material to ensure sufficient correction. It is typically placed throughout the vocal fold tissues, making precise correction of defects difficult. The major problem with currently available bioimplants is their unpredictable persistence. Injection of important extracellular matrix components such as hyaluronic acid (HA) is an attractive option because it is found in normal vocal fold lamina propria.55 Although HA is also prone to rapid resorption, it can be prepared with varying degrees of cross-linkage to facilitate persistence in the vocal fold; an example is the double cross-linked hyaluronan preparation Hylan B. There is unfortunately an inverse relationship between persistence and the amount of active HA available based on the extent of cross-linkage. Ongoing research is addressing the possible role of active substances that might attract favorable extracellular matrix in growth, and growth factors that induce production of favorable substances in the lamina propria. At this time there is a spectrum of effective injectable substances that have proven effective and safe. Each substance has a range of indications, unique physical characteristics, and optimal techniques to maximize results. There are also problems encountered with each. Table 14.1 gives a cursory outline of these features.
Management of Vocal Fold Incompetence with Vocal Fold Injectable Fillers
Vocal Fold Incompetence
Etiology
Patient Findings
Injection Laryngoplasty for Treatment of Vocal Fold Incompetence
Functional and Anatomic Goals
Injection Materials
Selection of Injection Materials
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree