MRI scans of the head. (a) Diffusion-weighted MRI scan demonstrated minimal restricted diffusion involving the left posterior limb of the internal capsule. (b) The perfusion-weighted MRI demonstrated a larger area of hypoperfusion deep within the left cerebral hemisphere, indicating a large mismatch volume ratio of ischemic brain parenchyma to infarction.
The anesthesiologist was prepared for the patient’s arrival at the neuroendovascular suite at 10:45 p.m. She was quickly transferred from the transport gurney to the procedure table and access to the right common femoral artery was achieved at 10:53 p.m. A 6F sheath was introduced into the femoral artery. Using an exchange length 0.035″ Glidewire (Terumo, Shibuya, Tokyo), the 6F sheath was then exchanged for an 8F Neuron MAX 088 guide catheter (Penumbra, Alameda, CA) and was advanced into the aortic arch. After removing the exchange length wire from the 8F guide catheter, a Neuron 5F Berenstein select catheter (Penumbra) loaded with a 0.035″ wire was introduced into the 8F guide catheter. The left common carotid artery was selected with fluoroscopic visualization and the 8F guide catheter was advanced into the left internal carotid artery (ICA). The 5F inner select catheter and 0.035″ wire were removed.
An angiogram of the left intracranial ICA distribution demonstrated a complete occlusion of the left mid M1 segment (Figure 8.2a). Using a roadmap technique, a 5Max ACE guide catheter (Penumbra) was advanced into the ICA over a Marksman microcatheter (Covidien, Dublin, Ireland) loaded with a Synchro-14 guidewire (Stryker, Kalamazoo, MI). Under fluoroscopic visualization, the guidewire was advanced through the left M1 thrombus. The microcatheter was then carefully advanced through the M1 thrombus over the guidewire, and the guidewire was removed.
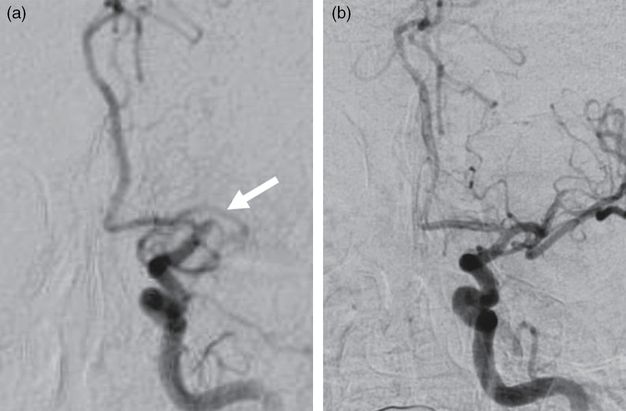
Anterior-posterior catheter angiograms of the left ICA before (a) and after (b) mechanical thrombectomy. The left M1 segment occlusion is indicated by the arrow.
An angiogram obtained by a microinjection through the microcatheter confirmed the microcatheter was distal to the thrombus. A 4 × 20 mm Solitaire Retriever stent (Covidien) was deployed across the M1 thrombus. The microcatheter was removed while the stent retriever was deployed. Continuous aspiration was applied to the 5Max ACE catheter, and the stent retriever was retracted after 2 minutes. An angiogram obtained of the left ICA demonstrated only partial recanalization. The stent retriever was used a second time resulting in complete recanalization of the left M1 segment at 11:10 p.m. (Figure 8.2b). The catheters were removed from the patient and the right femoral arteriotomy was closed with an 8F Angio-Seal closure device (St. Jude Medical, Saint Paul, MN).
The patient was admitted to the neuroscience intensive care unit (NSICU) for stroke management. Immediately after the patient was safely transferred to the NSICU, the neuro-interventionalist, neuro-interventional nurse, neuro-interventional radiology technician, and research coordinator met before leaving the hospital to debrief the case, discussing and documenting any issues encountered during the management of the case. This documentation was then later reviewed at a monthly stroke quality review conference to identify problems and potential solutions for future cases.
After the mechanical thrombectomy, the patient’s examination rapidly improved. On the following morning, she was alert and following commands with full strength in all of her extremities. Her NIHSS score improved from 16 to 1. The patient was evaluated for modifiable stroke risk factors, and she was started on simvastatin immediately and, after a 24-hour period after IV tPA administration, was started on a daily aspirin and prophylactic dose of enoxaparin for deep vein thrombosis (DVT) prevention. Cardiology was consulted due to her AF, which was rate controlled with propranolol. By hospital day 7, the patient was transferred to an acute rehabilitation facility with mild dysarthria and a slow gait that required assistance.
Discussion
Providing endovascular intervention for patients presenting with AIS does not start with a groin puncture, but rather begins with careful preparation and coordination among many different healthcare providers and organizations well before a stroke occurs. Efforts to provide improved stroke care delivery began after the approval of IV tPA by the US Food and Drug Administration (FDA) in 1996. Regional disparities in public awareness, acute stroke care teams, and stroke units were identified by the year 2000 and served as the motivation for a substantial reorganization of regional stroke care in the United States. By 2003, The Joint Commission (TJC) in the United States began to formally certify and recognize hospitals as primary stroke centers based on recommendations from the AHA/ASA, the National Stroke Association, and the Brain Attack Coalition (BAC). Primary stroke centers serve as regional focal points providing standardized care to patients that present with acute stroke in a timely manner. Primary stroke centers provide care to most patients with acute stroke, have the ability to provide some acute therapies such as IV tPA, and provide admission to a designated stroke unit. Table 8.1 summarizes the requirements defined by TJC for a primary stroke center designation [12]. Primary stroke centers have been shown to reduce stroke-related deaths by 14%, death or institutionalized care by 18%, and death or dependency by 18% [13].
Stroke center features | Primary stroke center | Comprehensive stoke center |
|---|---|---|
Neurology availability | Optional | Required |
Neurosurgical availability | Neurosurgical service available within 2 hours | Onsite neurosurgical service required |
Cerebrovascular surgery | ns | Required |
Stroke team | Required | Required |
Neuro-interventional capabilities | ns | Required |
Advanced practice nurse | ns | Required |
Written care protocols | Required | Required |
Emergency department | Required | Required |
Intensive care unit | ||
Stroke unit | Optional | Required |
Neurocritical care unit | Optional | Required |
Neuroimaging | Required | Required |
Rehabilitation therapy | Required | Required |
Speech/swallow assessment | ns | Required |
Performance measure collection and analysis | Required | Required |
Participation in clinical research | ns | Required |
ns: not specified.
Just as the need to rapidly administer IV tPA to patients with AIS within a strict therapeutic time period prompted the development of primary stroke centers, the evolution of mechanical thrombectomy technologies helped to provide an impetus to recognize comprehensive stroke center facilities. In 2012, TJC began to certify and recognize comprehensive stroke centers based on guidelines outlined by the BAC. In addition to the requirements for primary stroke center certification, designated comprehensive stroke centers have available resources needed to treat stroke patients who require a higher level of medical, surgical, and interventional care. This population includes large hemispheric ischemic strokes, intraparenchymal hemorrhages, and atraumatic subarachnoid hemorrhages. Requirements for a comprehensive stroke center designation (Table 8.1) include onsite, 24-hour coverage of specialized healthcare providers including vascular neurology, vascular neurosurgery, critical care medicine, and a neuro-interventionalist. The requirements also specify available infrastructure including neurointensive care unit, operating suite, and an interventional suite. Advanced neuroimaging that is available 24 hours a day is required of comprehensive stroke centers including perfusion imaging.
This case presentation exhibits the carefully orchestrated sequence of events that occur at a comprehensive stroke center once an acute stroke patient is identified at another community emergency department. Prior to the presentation of the stroke patient, representatives from the stoke team at the comprehensive stroke center met with and provided education regarding management of stroke patients to community ED personnel, including the ED physician who initially managed the patient from the case presentation. Thus, when the referral call was placed from the ED physician to the stroke neurologist taking call at the comprehensive stroke center, cooperation was already established and the decision to administer IV tPA at the community ED was made more efficiently after the clinical history and CT imaging was reviewed. Prior to the stroke presentation, a working relationship was also established with a local emergency transport service to ensure the patient was carefully monitored during the transfer and that regular ETAs were forwarded to the transfer center of the comprehensive stroke center, allowing the accepting stroke team to efficiently prepare for the patient’s arrival.
An essential concept illustrated by the case presentation is the importance of predefined responsibilities of each stroke team member. These predetermined roles helped streamline patient care, which was facilitated by constant communication of information among the stroke team members, the transfer center, and the transferring facility. For example, the neuro-interventional nurse being the only member to contact the outside facility’s ED nurse for report regarding the patient in-transit, who then informed the rest of the stroke team, epitomizes elimination of unwanted redundancy. These established responsibilities of each member are essential to provide efficient and expedient patient care.
The post-procedure debriefing following the interventional case is another important point illustrated by the case presentation. This step is crucial to identify and document difficulties that occurred during the management of the patient. These issues were then reviewed at a monthly quality control conference to remedy problems and implement solutions to avoid repeating the same difficulties with future patients. Lastly, the research coordinator continued to track outcomes based on ongoing data analysis to aid in implementing improvements.
Tip
Rapid identification and treatment of acute stroke is associated with improved clinical outcomes. Coordinated care is imperative.
Case 2. Periprocedural complications
Case description
A 41-year-old woman with morbid obesity, congestive heart failure (CHF), status post automatic implantable cardioverter defibrillator (AICD) placement, hypertension, type 2 diabetes mellitus, pulmonary hypertension, and sarcoidosis presented to a community ED with acute onset of difficulty speaking and right hemiplegia that started at 11:45 p.m. At the community hospital, her initial NIHSS score was 23. A CT scan of the head did not demonstrate an acute intracranial process. The ED physician initiated a stroke telemedicine consult at a comprehensive stroke center. After the patient’s presentation was reviewed with the stroke neurologist on-call, IV tPA was administered and arrangements for transfer were made.
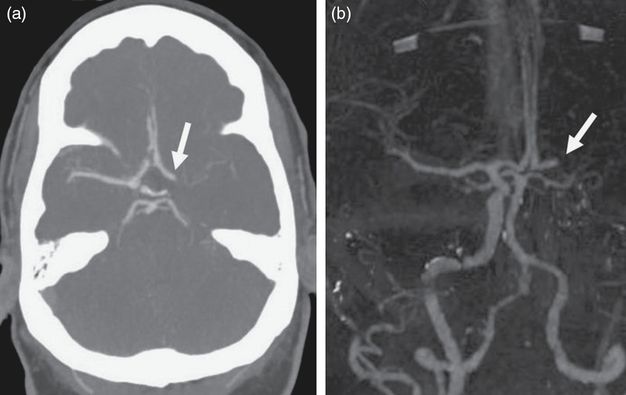
On arrival at the comprehensive stroke center at 3:40 a.m., almost 4 hours since the patient was last known to be normal, her NIHSS score remained 23. She was lethargic, requiring painful stimuli to remain awake. She had a global aphasia, left gaze deviation, right facial droop, and right hemiplegia. She did not follow commands, but had purposeful movements of her left arm and leg. Given the presence of the AICD, the patient was transferred to the CT scanner suite where a non-contrast CT scan showed a hypodensity within the deep left hemisphere. CT perfusion imaging of the head demonstrated decreased cerebral blood volume (CBV) and cerebral blood flow (CBF) within the left basal ganglia, left insula, and left temporal lobe consistent with cerebral infarction. The mean transit time (MTT) of the entire left middle cerebral artery (MCA) distribution was significantly increased, consistent with a large penumbra of ischemia within the remaining left MCA territory. A computed tomography angiography (CTA) scan of the head revealed an occlusion of the left ICA terminus with robust opacification of the left anterior cerebral artery (ACA) distribution from a patent anterior communicating artery (Figure 8.3) and some opacification of the distal left MCA distribution via retrograde leptomeningeal collateral circulation. The patient was then immediately transferred to the endovascular suite.
At 4:08 a.m., the patient arrived at the endovascular suite and was positioned supine on the procedure table. The patient’s airway could not be adequately protected while lying supine and general endotracheal tube anesthesia was started, ensuring that the systolic blood pressure remained around 140–160 mmHg. The patient was prepped and draped. A 6F sheath was inserted into the right common femoral artery. An exchange length 0.035″ Glidewire (Terumo) was introduced into the sheath. The sheath was exchanged for an 8F Neuron 088 guide catheter (Penumbra) and, under direct visualization, the guide catheter was advanced to the aortic arch. A Neuron 5F Berenstein select catheter over a 0.035″ Glidewire was used to help guide the guide catheter into the left ICA under fluoroscopic visualization. The 5F Berenstein select catheter and guidewire were removed.
An angiogram of a left ICA injection demonstrated complete occlusion of the supraclinoid segment of the left ICA (Figures 8.4a and 8.4b). A 5Max ACE catheter over a 0.014″ Synchro microwire (Stryker) was carefully advanced through the left ICA to engage the proximal portion of the thrombus. The guidewire was removed and continuous aspiration was applied to the catheter as the catheter was carefully advanced to the ICA terminus.
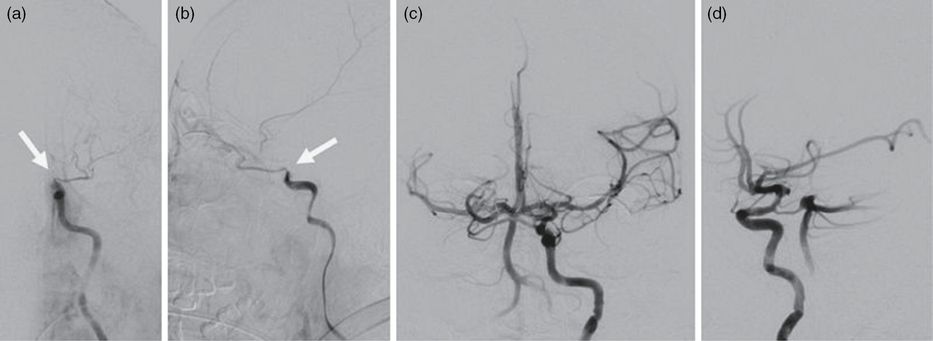
Anterior-posterior (a) and lateral (b) projections of a left ICA catheter angiogram demonstrating occlusion of the left ICA distal to the ophthalmic segment (arrows). Complete recanalization of the left ICA is demonstrated anterior-posterior (c) and lateral (d) in left ICA angiogram after mechanical thrombectomy.
The catheter was then withdrawn back into the cavernous ICA and an angiogram demonstrated complete recanalization of the left ICA, but a thrombus fragment remained in the left MCA. After a failed attempt to aspirate the MCA thrombus using aspiration through the catheter, a 0.025″ Velocity microcatheter (Penumbra) was loaded with a 0.014″ Synchro microwire (Stryker) and introduced into the 5Max catheter. The microcatheter was then advanced through the M1 segment passed the thrombus and a 6 × 20 mm Solitaire FR stent retriever (Covidien) was deployed across the clot. The microcatheter was removed. With aspiration applied to the 5Max catheter, the stent retriever was withdrawn after 2 minutes. The resulting angiogram of the left ICA at 4:50 a.m. demonstrated complete recanalization of the left anterior circulation (Figures 8.4c and 8.4d). The catheters were removed from the patient and the right femoral arteriotomy was closed with an Angio-Seal closure device (St. Jude Medical). The patient remained intubated at the end of the procedure and was admitted to the NSICU.
The patient’s examination was unchanged from her initial presentation when she arrived at the NSICU. She was intubated, had a left gaze deviation and her pupils were bilaterally equal and reactive. She still had right hemiplegia and purposeful movements of the left arm and leg. A new hyperdensity deep within the left hemisphere concerning for hemorrhagic conversion of infarcted brain parenchyma was noted on routine post-procedure CT imaging (Figure 8.5). A dual-energy CT scan of the head was then obtained, characterizing the hyperdensity as contrast media used during the intra-arterial (IA) recanalization rather than acute hemorrhage (Figure 8.6). The patient was medically optimized, and permissive hypertension was allowed, keeping the systolic blood pressure below 180 mmHg.
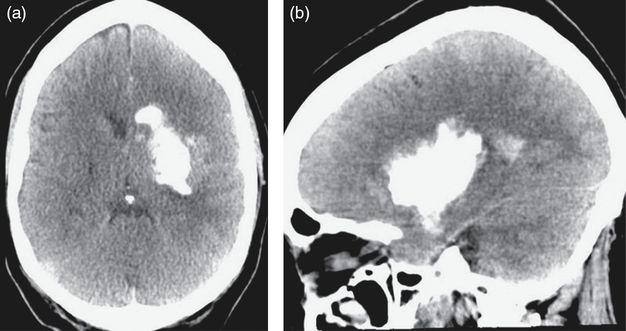
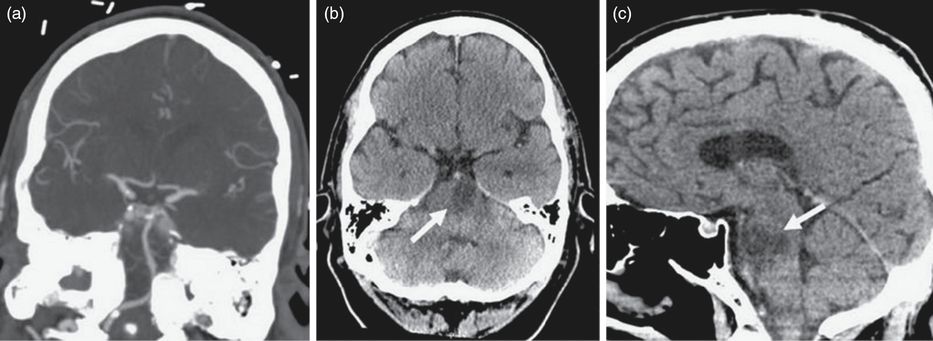
Axial (a) and sagittal (b) CT images of the head demonstrating a hyperdensity deep within the left hemisphere after a mechanical thrombectomy.
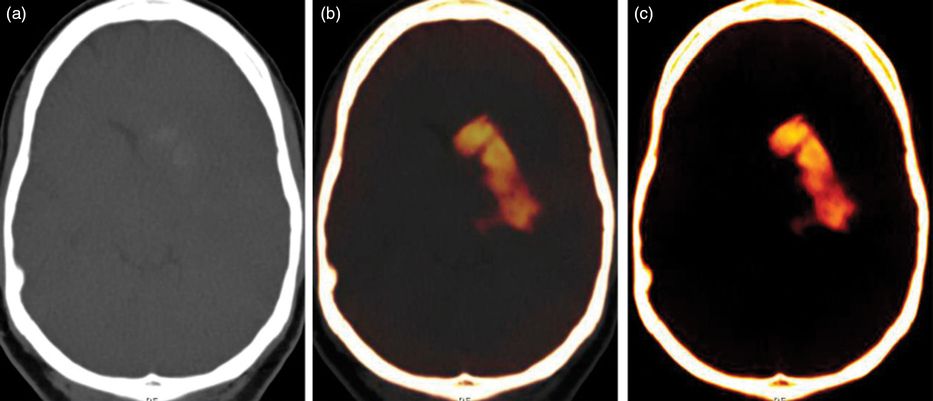
Dual-energy CT imaging. Based on the different attenuation characteristics of acute hemorrhage and iodine at two different X-ray spectra, a virtual non-contrast image (a), mixed (b), and iodine overlay map (c) was reconstructed demonstrating that the hyperdensity within the right cerebral hemisphere was iodine contrast media rather than hemorrhage.
By 1:45 a.m. on the following morning, the patient’s neurologic examination acutely changed. The right pupil was found to be dilated and non-reactive, and she exhibited extensor posturing. An emergent CT scan showed that the left cerebral hyperdensity was stable in size. There was increased cerebral edema of the left hemisphere with a resulting midline shift indicative of a malignant MCA infarction. Following the CT scan, 116 g of IV mannitol was administered and IV 3% NaCl with goal sodium of 155–165 mmol/L saline was started. The neurosurgical service recommended an emergent left hemicraniectomy. After carefully reviewing the risks and benefits of the surgical procedure and the long-term prognosis of the patient, the patient’s next of kin, her mother, elected to decline both surgical intervention and further medical management in favor of comfort care measures. A do-not-resuscitate order was initiated. Later in the day, the AICD was deactivated, ventilation support was stopped, and the patient expired.
Discussion
Improvements of technology and greater experience have made periprocedural complications associated with endovascular intervention of AIS equivalent to medical management of AIS, including the administration of IV tPA [6]. The IMS III trial, for instance, showed no significant difference for mortality, symptomatic intracerebral hemorrhage (ICH), or other non-intracerebral bleeding complications between the endovascular therapy treatment arm and the IV tPA treatment arm [6]. Periprocedural complications of IA stroke therapy can be broadly classified into five categories: vascular access complications, complications of medication and contrast media, systemic complications, complications associated with anesthesia, and device-related complications [14].
Hemorrhagic complications, which significantly increase morbidity and mortality, are the most frequent complications and are usually associated with the use of thrombolytic agents or reperfusion injury [14]. The IMS III trial reported a 6.2% incidence of symptomatic ICH within 30 hours of endovascular therapy for stroke [6]. Clinical trials of mechanical thrombectomy for treatment of AIS have established a safe treatment window of 5–8 hours from the onset of initial stroke symptoms. ICH most likely occurs as the blood–brain barrier (BBB) breaks down resulting in reperfusion injury, but may represent a technical error such as a vessel perforation caused by a catheter, microwire, or device. Careful patient selection for endovascular intervention is important in order to identify patients at higher risk of hemorrhagic complications. Extent of infarction demonstrated on initial neuroimaging [15] and time interval from symptom onset to recanalization are predictors of hemorrhagic transformation. Advanced neuroimaging techniques such as CT perfusion, MRI, and MRI perfusion imaging capable of defining the extent of core infarction relative to the volume of ischemic penumbra may improve patient selection for endovascular intervention by establishing safe, objective pathophysiologic parameters for intervention, instead of relying on clinical history that may at times be unreliable.
Early recognition of ICH is crucial. Many contemporary endovascular suites have the capability to obtain flat panel detector CT imaging with acquisition quality comparable to multidetector CT imaging if ICH is clinically suspected prior to conclusion of an endovascular procedure. During angiography, the mass effect of an ICH may also become evident as distortions of the intracranial vascular anatomy or visualized extravasation of contrast media. As illustrated by the case presentation, leakage of iodine contrast media into brain parenchyma resulting from increased permeability of the BBB documented on routine post-procedure CT imaging may be mistaken for periprocedural ICH.
Continuing anticoagulation therapy is dependent on early differentiation of contrast leakage versus ICH. Distinguishing contrast material leakage from ICH is difficult because of the similar Hounsfield densities of iodine and acute hemorrhage. Serial imaging is necessary to demonstrate gradual resolution of iodine contrast from an increase of stable volume of ICH. A dual-energy CT scan can differentiate iodine from hemorrhage with a single scan [16]. In our practice, management of intracranial hemorrhage includes early surgical decompression when indicated, placement of an external ventricular drain (EVD) for evidence of hydrocephalus or suspected elevated intracranial pressure (ICP), and, although not evidence-based, consideration for prophylactic antiepileptic therapy.
Other periprocedural complications of endovascular treatment for AIS are less common. Iatrogenic arterial dissection is a rare complication with a reported incidence of 0.4% [17]. Angioplasty with possible stenting may be required for flow-limiting dissections; otherwise, small arterial dissections may be managed with antiplatelet therapy. Distal emboli can occur during a mechanical thrombectomy procedure as a thrombus or thrombus fragment migrates into smaller branches during manipulation. The use of aspiration either through large-bore soft catheters [18] or balloon guide catheters [19] has decreased the incidence of distal emboli. The incidence of air emboli is less than 0.1% and can be avoided with meticulous technique during the endovascular procedure [20]. Published recommendations for the management of symptomatic air embolism are limited. Elevated mean arterial pressure greater than 100 mmHg, ventilation with 100% oxygen, IA verapamil infusion, and prophylactic antiepileptic therapy should be considered for symptomatic air embolism. Arterial vasospasm can occur after manipulation of catheters, wires, and devices within the intracranial vasculature and can be managed with IA vasodilator infusion or angioplasty.
Tip
Symptomatic ICH is the most common periprocedural complication associated with endovascular intervention for acute stroke. Early differentiation of iodine leaking through a disrupted BBB from true hemorrhage dictates management strategy and can be accomplished quickly with dual-energy CT imaging. Other periprocedural complications are rare. Neuro-interventionalists should be aware of these rare complications and familiar with treatment strategies.
Case 3. Basilar artery occlusion
Case description
A 58-year-old man with atrial fibrillation, coronary heart disease status post a four-vessel bypass 7 years prior, a minimally invasive maze procedure, and placement of an implantable cardioverter defibrillator 6 years prior, abruptly developed severe nausea and emesis at approximately 5:00 p.m. on the day prior to presentation. He had been noncompliant on his oral anticoagulation with rivaroxaban secondary to the patient’s confusion with his own prescriptions and has been off of any oral anticoagulation for a long period of time. He did not initially seek medical attention after onset of his symptoms. At approximately 1:00 a.m. after a restless night, his symptoms progressed to a right hemiplegia, prompting him to contact the EMS who transported the patient to a community hospital. There, a CT scan of the head demonstrated a hyperdense basilar artery (BA) suggestive of a possible embolic occlusion. A stroke telemedicine consult was initiated at a comprehensive stroke center and the patient’s initial NIHSS score was determined to be 12 due to right hemiplegia and dysarthria. He was not a candidate for IV tPA since onset of symptoms was well beyond the 4½ hour window of time for systemic therapy, and he was transported to a comprehensive stroke center for possible endovascular intervention.
On arrival at the comprehensive stroke center at 6:30 a.m., the patient became increasingly somnolent and his NIHSS score increased to 13. A CT angiogram of the head demonstrated complete occlusion of the intradural segment of the right vertebral artery (VA) and proximal BA. The distal third of the BA was supplied by a patent right posterior communicating artery (Figure 8.7a). The entire course of the left vertebral artery was not visualized, presumably representing a congenitally absent left VA. The patient had a generalized tonic-clonic seizure during the acquisition of the CT angiogram and received a 1 gram IV bolus of levetiracetam and was promptly brought to the endovascular suite.
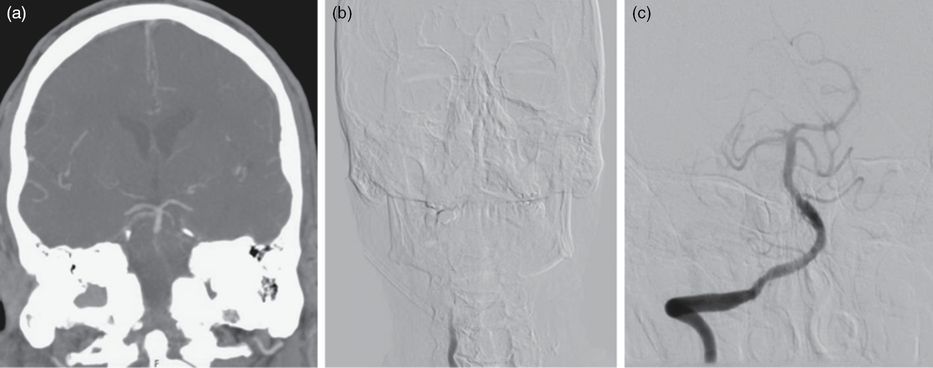
(a) Coronal CT angiogram demonstrating vertebral-basilar occlusion with retrograde filling of the distal BA. (b) Anterior-posterior catheter angiogram of a right VA injection demonstrating occlusion. (c) Anterior-posterior catheter angiogram of a right VA injection after mechanical thrombectomy.
In the endovascular suite, the patient was quickly positioned, prepped, and draped. Access to the right common femoral artery was obtained with a 6F sheath using local anesthesia and mild sedation. An Envoy DA guide catheter (Codman Neuro, Raynham, MA) with a 0.035″ Glidewire (Terumo) was introduced into the sheath and the right vertebral artery was selected under fluoroscopic guidance. An angiogram showed delayed filling of the proximal right VA indicative of a distal occlusion of the distal VA (Figure 8.7b). Through the guide catheter, a Trevo Pro 18 microcatheter (Stryker) was advanced through the thrombus within the right VA extending into the BA over a Synchro-14 microwire (Stryker). A 4 × 20 mm Trevo XP ProVue Retriever stent (Stryker) was deployed across the thrombus. After 5 minutes, aspiration was applied to the guide catheter and the stent was withdrawn through the guide. An angiogram demonstrated complete opacification of the posterior circulation (Figure 8.7c). The guide catheter was withdrawn and the groin puncture site was closed with a 6F Angio-Seal device (St. Jude Medical) after the sheath was removed. The patient was transferred to the NSICU afterward.
The patient was medically optimized and spent a total of 5 days in the NSICU. Full recanalization of the right vertebral artery and basilar artery was demonstrated on a post-procedure CT angiogram (Figure 8.8a). However, a non-contrast CT scan did show a left pontine hypodensity indicative of a brainstem infarction (Figures 8.8b and 8.8c). The patient was restarted on his anticoagulation in the form of rivaroxaban 10 days after his stroke. Over his hospitalization course, he gained some strength in his right leg, but his dysarthria remained unchanged. He was deemed to be an excellent candidate for physical rehabilitation and was ultimately discharged to an inpatient rehabilitation center. His discharge medications included levetiracetam, aspirin, prophylactic dose of enoxaparin, and rivaroxaban. At the time of discharge, his NIHSS score was 11.
At a 3-month outpatient follow-up visit, he was requiring some assistance at home from his sister. His left hemiparesis had improved and he was able to independently ambulate with a cane. He continued to receive physical therapy and occupational therapy twice a week. Although he declined speech therapy, his dysarthria had improved since discharge.
Discussion
Posterior circulation ischemic strokes account for nearly 20% of all strokes. A number of characteristics distinguish AIS involving the posterior circulation from anterior circulation stroke. The brainstem is a critical neurologic structure that receives arterial supply mostly from the BA, the main vessel of the posterior circulation. Acute BA occlusion (BAO) is a catastrophic event with a grave prognosis if left untreated. Acute BAO treated with supportive therapy along with antiplatelets, antithrombotics, or a combination of the two has an associated 40% mortality and 65% severe disability for survivors [21].
The BA can potentially receive additional collateral circulation through patent posterior communicating arteries aside from leptomeningeal collateral circulation analogous to ischemic stroke involving the anterior circulation. This potential collateral circulation from the circle of Willis, along with other factors including the level of occlusion and the extent of the thrombus, makes the clinical presentation of BAO more variable. Also, greater than 60% of patients with BAO have prodromal symptoms including vertigo, nausea, headache, hemiparesis, and diplopia weeks prior to onset of stroke [22]. These prodromata prior to BAO may confound the timing for effective intervention.
Most large clinical trials for mechanical thrombectomy as a treatment for cerebrovascular occlusion to date have included mainly anterior circulation stroke and established a safe treatment window of 5–8 hours from the onset of any stroke symptoms. A recent large prospective observation registry of BAO demonstrated that the time period for optimal, safe intervention for BAO should begin with the onset of severe neurologic deficit clinically consistent with BAO, rather than the start of any minor stroke symptoms as would be considered for anterior circulation stroke onset [23].
The case vignette illustrates a typical presentation of BAO with non-focal symptoms including severe nausea and emesis, which presented 12 hours prior to admission to a comprehensive stroke center. As per the indications of the stent retriever used in the case, the patient would not have been a candidate for mechanical thrombectomy as the patient’s stroke symptoms were over 8 hours. Ultimately, the patient tolerated the procedure well and being made a favorable recovery, returning to his home and being able to ambulate independently with a cane. This case supports endovascular intervention for this disease process that bears a potentially devastating prognosis.
Case 4. Pediatric acute ischemic stroke
Case description
A 2-year-old boy with history of hypoplastic left heart syndrome requiring multiple cardiothoracic reconstructive surgeries and heterozygosity for the prothrombin G20210A mutation managed with warfarin presented with acute left hemiplegia. At baseline, the patient was without neurologic deficits. At 2:30 p.m. on the day of presentation, his mother initially noticed that his gait progressively worsened from walking normally, to having a limp on the left, to becoming non-ambulatory. She attributed this to her son being defiant, and the child was set down for a nap. Fifteen minutes later, he awoke with a persistent left hemiplegia. The patient’s mother brought him to the ED for evaluation.
On presentation to the ED, the patient was alert and appropriately irritable. He had a left facial droop and left hemiplegia. He did not withdraw to noxious stimuli applied to the left arm or leg. His INR was found to be 1.57, which was subtherapeutic to his goal of 1.8–2.5. A CT scan of the head demonstrated an area of hypodensity in the right hemisphere concerning for an AIS. Consults were made to the stroke neurology service and neuro-interventionalist. The patient was electively intubated. An MRI/MRA of the head demonstrated an occlusion of the proximal right M1 segment with restricted diffusion within the right lentiform nucleus and posterior limb of the internal capsule (Figure 8.9). After the MRI, the patient was transferred to the endovascular suite.
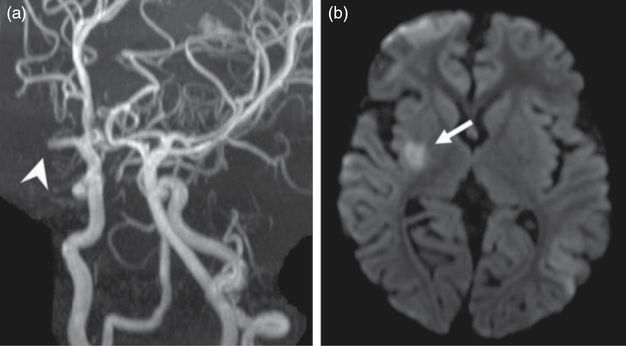
(a) MRA demonstrating an occlusion of the right M1 segment (arrowhead). (b) Diffusion-weighted MRI scan showing restricted diffusion within the right lentiform nucleus and posterior limb of the internal capsule (arrow).
Under general endotracheal tube anesthesia, a 10 cm 4F sheath was inserted into the right common femoral artery using a micropuncture technique. A 65-cm 4F angled Glidecath (Terumo) was introduced into the sheath and advanced to the aortic arch over a 0.035″ Glidewire (Terumo) under direct visualization. The right common carotid artery was selected without difficulty despite the previous aortic arch reconstruction. The right ICA was selected using a roadmap technique and the guidewire was withdrawn. An angiogram obtained of the right ICA confirmed the occlusion of the proximal M1 segment (Figure 8.10a). A Trevo Pro 14 microcatheter (Stryker) was loaded with a 0.014″ Synchro microwire (Stryker) and introduced into the 4F guide catheter. The microwire was carefully advanced through the M1 thrombus followed by the microcatheter. The microwire was withdrawn and successful positioning of the microcatheter completely through the thrombus was confirmed by an angiogram obtained from a microinjection through the microcatheter (Figure 8.10b). The 3 × 20 mm Trevo XP ProVue Retriever stent (Stryker) was then deployed through the thrombus (Figure 8.10c). After allowing the stent retriever to incorporate into the clot, the stent and microcatheter were retracted as a unit back into the 4F guide catheter. A subsequent angiogram of the right ICA demonstrated that the M1 segment remained occluded and a fragment of clot had migrated into the right ACA (Figure 8.11a).
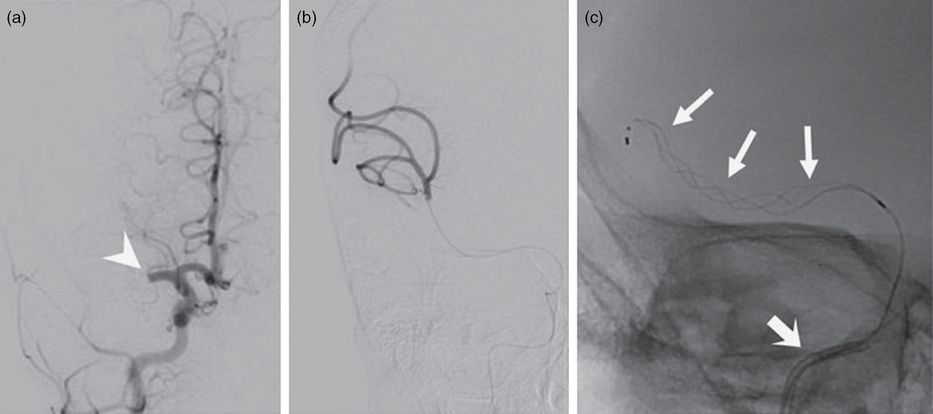
(a) Anterior-posterior catheter angiogram of a right ICA injection demonstrating a right M1 segment occlusion (arrowhead). (b) Microinjection angiogram through a microcatheter passed though the right M1 thrombus, confirming that the distal end of the catheter is distal to the thrombus. (c) Anterior-posterior fluoroscopic image demonstrating a stent retriever (arrows) deployed within the right M1 segment. The 4F guide catheter is visible within the intracranial ICA (thick arrow).
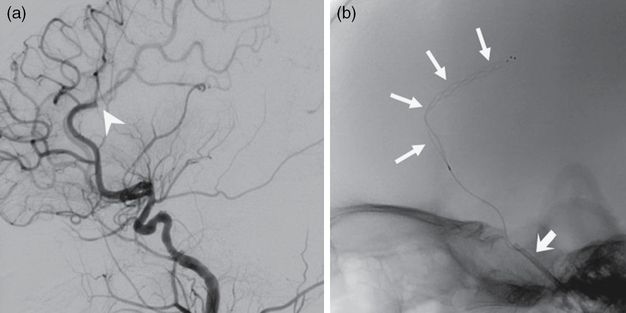
(a) Lateral catheter angiogram of a right ICA injection showing an ACA occlusion (arrowhead). (b) Lateral fluoroscopic image depicting a stent retriever (arrows) within the right ACA. The 4F guide catheter is seen within the intracranial ICA (thick arrow).
After the initial failed thrombectomy attempt using the 3 × 2 mm stent retriever, a Trevo Pro 18 microcatheter (Stryker) loaded with a 0.014″ microwire was introduced into the 4F guide catheter. Again, the microwire was carefully advanced through the M1 thrombus and the microcatheter was then advanced past the thrombus over the wire. A 4 × 20 mm Trevo XP ProVue Retriever stent (Stryker) was then deployed across the M1 thrombus. While the stent retriever incorporated into the clot over 5 minutes, the microcatheter was withdrawn, increasing the space between the stent retriever pusher wire and the inner wall of the 4F guide catheter. While gentle aspiration was applied to the 4F guide catheter, the stent retriever was then withdrawn through the 4F guide catheter. The resulting angiogram of the right ICA demonstrated a partial recanalization of the right M1 segment. Ultimately, a third pass using a larger 4 × 20 stent retriever and aspiration through the 4F guide catheter successfully recanalized the right M1 segment (Figure 8.12a).
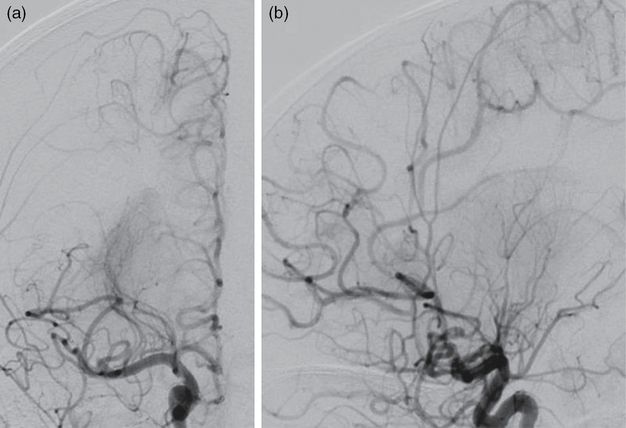
Anterior-posterior (a) and lateral (b) catheter angiogram projections of a right ICA injection after mechanical thrombectomy demonstrating recanalization of the right MCA and right ACA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








