Chapter 35 Disorders of Glycosylation
Eukaryotic cells synthesize hundreds of types of sugar chains called glycans, which function within the cell, at the cell surface, and beyond. Within the cell, glycans influence protein folding, stability, turnover, and intracellular trafficking [Varki et al., 2009]. At the cell surface, they influence or determine cell-cell binding, receptor-ligand interactions, assembly of signaling complexes, binding to the extracellular matrix, tissue pattern formation, trafficking of lymphocytes, and much more [Varki et al., 2009]. The same glycan can function differently on different proteins or in different settings. This three-dimensional complexity makes understanding the roles of glycans challenging but provides the body with an extraordinarily sensitive fine-tuning mechanism for many physiologic functions. It is not surprising that disrupting normal glycosylation causes moderate to severe pathology in multiple human organ systems.
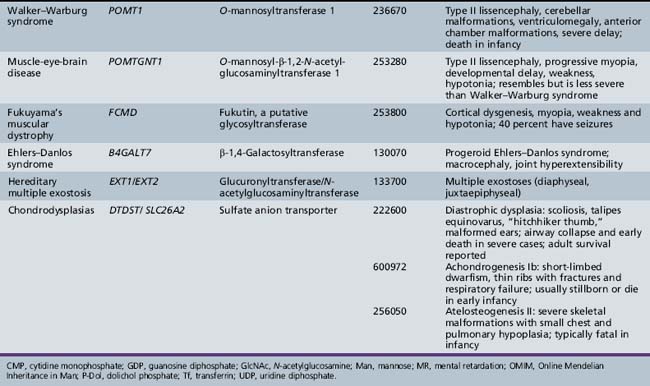
More than 40 rare inherited disorders of glycan biosynthesis have been identified [Jaeken et al., 2008; Grunewald, 2007; Freeze, 2007; Marquardt and Denecke, 2003]. Most of these are the newly defined congenital disorders of glycosylation (previously called carbohydrate-deficient glycoprotein syndrome and disialotransferrin developmental deficiency syndrome). Others, such as muscle-eye-brain disease and Walker–Warburg syndrome, were well known, but elucidation of their relation to glycosylation has provided new insights into their pathophysiology and opens possibilities for therapy.
N-Linked Glycosylation
Overview
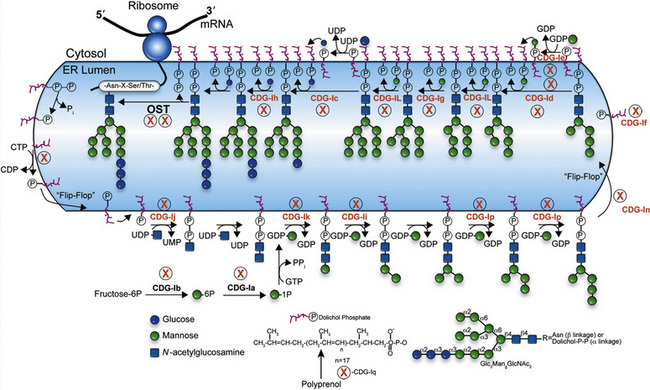
Sugar chains are added to newly synthesized proteins in the lumen of the endoplasmic reticulum; they are quickly and extensively remodeled there, and later in the Golgi apparatus. All eukaryotic cells make a 14-sugar lipid-linked oligosaccharide in the endoplasmic reticulum membrane that is composed of Man, GlcNAc, and glucose (Glc). This entire chain is transferred to Asn within an Asn-X-Thr/Ser/Cys [Zielinska et al., 2010] consensus sequence (X is any amino acid except proline) as newly made proteins emerge from the ribosome into the endoplasmic reticulum lumen. Forty or more genes are required to synthesize and transfer this glycan to proteins [Freeze, 2001b].
Extensive remodeling begins soon after sugar chain transfer. Up to two-thirds of the original lipid-linked oligosaccharide glycan is discarded, and 6–15 other sugar units are added back to create a dazzling array of sugar chains. Why generate this complex process? The initial glycan helps proteins fold and also provides important checkpoints for monitoring of proper protein folding in the endoplasmic reticulum [Parodi, 2000]. The addition of more sugars in the Golgi usually imparts greater specificity to the sugar chain function. Understanding the biosynthetic pathways is important for appreciating the nature of the defects.
Biosynthesis
Individual monosaccharides are synthesized from glucose, derived from the diet, or salvaged from degraded glycans, and must be activated to their nucleotide sugar derivatives [Freeze, 1999]. Figure 35-1 depicts an abbreviated version of the known monosaccharide pathways. We employ widely used standard symbols to denote the different sugars [Varki et al., 2009]. Note that phosphorylation of the monosaccharide is the first step, and some pathways interconvert phosphorylated forms, such as Man-6-P→Man-1-P. Also, several alternative routes generate uridine diphosphate (UDP)-GlcNAc, UDP-galactose (Gal), and guanosine diphosphate (GDP)-fucose (Fuc). For some types of glycosylation, the nucleotide sugar donates the sugar to a lipid carrier derived from the polyprenol, dolichol phosphate (P-Dol) [Schenk et al., 2001a]. These products include Man-P-Dol and Glc-P-Dol. Dolichol itself is made from polyprenols using a specific reductase [Cantagrel et al., 2010].
N-linked Glycan Biosynthesis
Biosynthesis of the lipid precursor oligosaccharide involves a series of steps and enzymes that add sugars in specific linkages and in a specific order [Freeze, 2001a]. It begins with P-Dol + UDP-GlcNAc forming GlcNAc-P-P-Dol on the cytosolic face of the endoplasmic reticulum. Another UDP-GlcNAc donates a second GlcNAc using a different GlcNAc transferase, and this is followed by the addition of five Man units derived from GDP-Man. At this point, a “flippase” reorients the entire molecule from the cytosolic face into the endoplasmic reticulum lumen. A series of Man transferases use Man-P-Dol to add four more Man units, making a three-branched structure. Three glucosyltransferases sequentially add Glc from Glc-P-Dol to one branch to complete the sugar chain. This 14-sugar unit molecule is the optimal substrate for the multisubunit oligosaccharyl transferase (OST) complex that recognizes the Asn-X-Thr/Ser consensus sequence on the protein and adds the sugar chain to Asn. Recent studies indicate that some Asn-X-Cys sites can also be glycosylated [Zielinska et al., 2010; Ostasiewicz et al., 2010]. Truncated glycans are poorly transferred and sometimes fail to occupy normal glycosylation sites. Following glycan transfer, P-P-Dol is converted back to P-Dol and then to Dol. Recycling of these carriers is extensive.
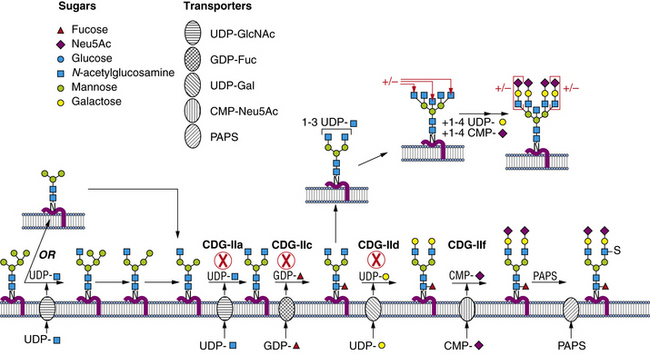
Within a few minutes of transfer to protein, the sugar chain is processed (Figure 35-2; see also Figure 35-1). A set of two glucosidases removes the Glc units. For some proteins, processing stops here, but for the great majority, a mannosidase in the endoplasmic reticulum removes a single Man unit. The proteins are carried to the Golgi, where another mannosidase called Golgi mannosidase I removes up to three more Man units. At this point, processing starts to add sugars. UDP-GlcNAc donates a GlcNAc to one branch, making the sugar chain an acceptable substrate for Golgi mannosidase II, which removes an additional two Man units. This process clears the way for the addition of a second GlcNAc to a Man on the remaining branch. Depending on the protein, up to three more GlcNAc transferases add GlcNAc units to the Man residues in a specific order to initiate up to five branches. These branches are then extended with Gal, derived from UDP-Gal, and sialic acid (Sia), derived from cytidine monophosphate (CMP)-Sia. In some cases, GDP-Fuc donates a Fuc to one or more of the branches or to the GlcNAc linked to the protein. In other cases, sulfates, phosphates, or glucuronic acids are added to selected sugars. Individual branches may accumulate several repeating units of Galβ1,4GlcNAc before being capped by Sia. These reactions occur in selected cisternae of the Golgi, and each nucleotide sugar donor must be translocated from its origin in the cytoplasm or nucleus to the Golgi by a substrate-selective transporter [Gerardy-Schahn et al., 2001].
Enzymes and Cell Biology
The sugar-activating enzymes reside in either the cytoplasm or the nucleus. The N-linked biosynthetic enzymes all are in the endoplasmic reticulum or Golgi, with their active sites situated in the expected orientation. Most of the glycosyltransferases used for building the lipid-linked oligosaccharide and the Golgi transporters span the membrane many times [Ishida and Kawakita, 2004]. Most enzymes participating in glycan processing are attached to the membrane by a single membrane span. The transferases and nucleotide sugar transporters are constantly recycled in the dynamic Golgi to maintain their correct relationship to the maturing glycoproteins as they pass through the Golgi. Therefore, correct trafficking of the biosynthetic machinery is essential for optimal function.
Congenital Disorders of Glycosylation
Glycosylation is complex, and its disorders do not always lend themselves to phenotypic or symptomatic pigeonholing. Congenital disorders of glycosylation (CDG) nomenclature is still evolving as attempts are made to design a systematic, clinically useful system based on biochemical origins. Until 2008, CDGs resulting from defects in lipid-linked oligosaccharide biosynthesis and transfer to protein were defined as group I. The remaining CDGs that affect the biosynthesis and processing of the protein-bound sugar chains constituted group II. Individual disorders in group I or II were assigned lower-case letters (designating types, as noted earlier) when the genetic defect is proved. Several reviews provide comprehensive and up-to-date perspectives [Vodopiutz and Bodamer, 2008; Jaeken and Matthijs, 2007; Grunewald, 2007; Freeze, 2007; Marquardt and Denecke, 2003]. In 2008 a simplification of the nomenclature was proposed, as the growth in the number of disorders had produced a confusing series of letters and numbers that did not help the clinician or researcher. In the new system, diseases will be grouped in four categories: disorders of protein N-glycosylation, disorders of protein O-glycosylation, disorders of glycosphingolipid and glycosylphosphatidylinositol anchor glycosylation, and disorders of multiple glycosylation pathways. Individual disorders will be listed by the gene involved and, in parallel, its protein product. The authors suggest that the previous CDG designation may be listed in parentheses during the transitional period as the new nomenclature is adopted [Jaeken et al., 2008].
Diagnosis
In most patients with CDGs the diagnosis is straightforward and is based on the analysis of serum transferrin isoforms. Several methods are suitable and commercially available. These include isoelectric focusing [Freeze, 2001a; Jaeken and Matthijs, 2001], mass spectrometry [Wada, 2007; Kleinert et al., 2003; Lacey et al., 2001], zone electrophoresis, and high-pressure liquid chromatography [Quintana et al., 2009]. Electrospray ionization-mass spectrometry [Babovic and O’Brien, 2007] is the most informative because it easily distinguishes the absence of entire sugar chains, which characterizes group I CDGs, from the absence of one or more individual monosaccharide units typical of group II CDGs. The predominant isoform in normal transferrin has two sugar chains, each containing two negatively charged Sia molecules, designated tetrasialotransferrin. In group I disorders, isoelectric focusing analysis demonstrates loss of one or both entire glycan chains, producing “disialotransferrin” or “asialotransferrin,” respectively, but clearly this is an incomplete description. By contrast, electrospray ionization-mass spectrometry demonstrates that the loss of one or two chains reduces the mass by about 2200 or 4400 mass units, respectively. In group II disorders, isoelectric focusing may distinguish among “tri-,” “di-,” “mono-,” and “asialo” forms, reflecting variable loss of Sia, whereas electrospray ionization-mass spectrometry can indicate specific loss of Sia alone or combinations of Sia with additional sugars. This distinction is essential for determining assignment to group I or group II, and provides a signpost for specific identification of the defect. The single-step electrospray ionization-mass spectrometry technique can be accomplished using less than 5 mL of blood in less than 30 minutes [Lacey et al., 2001; Babovic and O’Brien, 2007]. This technique is therefore suitable for large-scale population screening for CDGs.
Transferrin isoform analysis produces few false-positive results. Uncontrolled fructose and galactose intolerance and recent heavy alcohol consumption produce a pattern typical of group I disorders [Kleinert et al., 2003]. In apparently rare instances, patients with genetically confirmed CDGs exhibited normal transferrin isoelectric focusing profiles, and in some patients, previously abnormal patterns normalized in preadolescence [Dupre et al., 2001; Fletcher et al., 2000]. Thus, a normal transferrin pattern should not exclude follow-up testing. Healthy neonates sometimes have a slightly abnormal transferrin pattern, which normalizes within a few weeks. Suspicious results in neonates should be repeated.
Until recently, specific diagnosis of the defect usually involved enzymatic, biochemical, and genetic analysis of the patient’s fibroblasts or leukocytes. Relatively few laboratories performed these analyses. With routine gene sequencing becoming more cost-effective, some genetic centers and commercial laboratories now offer various CDG gene diagnostic panels. In the coming years, decreasing costs, improved sequencing technology, and better informatics are likely to make this approach the first option. Prenatal testing is available for confirmed at-risk families [Matthijs et al., 1998, 2004].
General Clinical Features
About 1000 CDG patients have been identified, presenting with multiple organ dysfunction [Haeuptle and Hennet, 2009]. Patients with CDGs have protean presentations that, in some cases, may mimic mitochondrial (oxidative phosphorylation) disorders [Briones et al., 2001], but lack a history of maternal inheritance. An informal survey of CDG-affected families indicated that earlier nonspecific diagnoses frequently included a metabolic defect or cerebral palsy. Most patients first present to pediatric neurology or metabolic clinics. They frequently have combinations of liver, gastrointestinal, and coagulation disturbances [Jaeken and Matthijs, 2001; Marquardt and Denecke, 2003]. The possibility of a CDG should be investigated in any child presenting with developmental delay, seizures, hearing loss [Jaeken et al., 2009] or strabismus, particularly if any of these manifestations is accompanied by abnormal coagulation, liver dysfunction, or a gastrointestinal disorder. Most affected children are hypotonic and demonstrate failure to thrive.
Specific Disorders
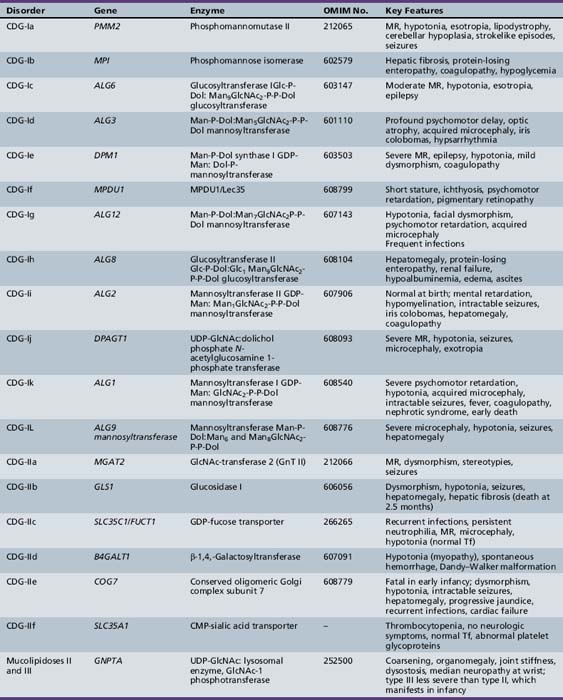
Table 35-1 summarizes the known CDG defects, utilizing the classification scheme proposed in 2008. This includes the mutated genes and major signs and symptoms. The known defects cover every aspect of the N-linked biosynthetic pathway. Activation or presentation of precursors (PMM2, PMI, DPM1, DPM3 MPDU1 [CDG-Ia, Ib, Ie, If]), glycosyltransferases for lipid-linked oligosaccharide biosynthesis (ALG6, NOT56L, ALG12, ALG8, ALG2, DPAGT1, HMT1, DOBD1 [CDG-Ic, Id, Ig, Ih, Ii, Ij, Ik, Il]), glycosidases that trim the protein-bound sugar chain (GLS1 [CDG-IIb]), Golgi-localized nucleotide sugar transporters (SLC35C1, SLC35A1 [CDG-IIc and IIf]), and glycosyltransferases that extend the trimmed chain (MGAT2, BG4ALT1 [CDG-IIa and IId]). DK1 (CDG-Im) impairs dolichol kinase function and impairs the final step of the de novo synthesis of dolichol phosphate [Denecke and Kranz, 2009]. SRD5A3 encodes the α-reductase that converts various polyprenols to dolichols [Cantagrel et al., 2010]. The conserved eight-subunit oligomeric Golgi (COG) complex that binds to the cytoplasmic face of the Golgi is needed for intra-Golgi or Golgi to endoplasmic reticulum retro-trafficking of multiple resident glycosyltransferases and nucleotide sugar transporters. Disorganized trafficking impairs multiple glycosylation pathways [Ungar et al., 2002]. Defects have now been identified in COG7, COG1, COG4 [CDG-IIe, IIg, IIj], COG8, COG5 and COG6 [Lübbehusen et al., 2010; Foulquier, 2009]. Appreciation of the importance of Golgi homeostasis in glycosylation led to the discovery of another disorder caused by mutations in a subunit of a vacuolar H+/ATPase that maintains appropriate pH of various organelles within the endocytic and exocytic pathways [Marshansky and Futai, 2008; Kornak et al., 2008; Guillard et al., 2009]. The intravesicular pH progressively decreases from the endoplasmic reticulum to Golgi, endosomes, and, finally, lysosomes.
The remainder of this chapter will focus on CDGs with significant neurologic manifestations.
Defects in Protein N-glycosylation
PMM2 (CDG-Ia)
PMM2 (CDG-Ia) is the best-known and most frequently recognized form of CDGs, first reported by Jaeken and co-workers in 1980 [Jaeken et al., 1980]. The defective gene was identified in 1995 as PMM2, which encodes the phosphomannomutase (PMM) that converts Man-6-P → Man-1-P. This defect results in insufficient production of lipid-linked oligosaccharide, leading to empty glycosylation sites. More than 800 patients are known worldwide, and more than 100 mutations have been cataloged [Barone et al., 2008; Jaeken, 2003; Matthijs et al., 2000; Haeuptle and Hennet, 2009].
Hagberg and associates described four stages of the typical (severe) phenotype [Hagberg et al., 1993]. The first is the infantile phase, marked by various combinations of dysmorphism, abnormal fat distribution (supragluteal and vulval fat pads, focal lipoatrophy), inverted nipples, cryptorchidism, esotropia, recurrent infections, cardiomyopathy or pericardial effusions, coagulopathies, nephrotic syndrome, hypothyroidism, life-threatening episodes of hepatic failure, and unexplained coma. As many as 20 percent of infants with CDG-Ia succumb in this phase [Jaeken and Matthijs, 2001]. In the second phase (comprising the remainder of the first decade), children experience seizures and strokelike episodes, often precipitated by intercurrent infections. The third phase (in the second decade of life) is marked by slowly progressive cerebellar ataxia and limb wasting, and by progressive visual loss secondary to pigmentary retinopathy. Adult survivors have moderate mental retardation with severe ataxia and hypogonadism, with or without skeletal deformities. Presentations are highly variable. In one girl with CDG-Ia, findings on computed tomography (CT) of the head were normal at 9 months, but subsequent imaging studies demonstrated progressive atrophy [Mader et al., 2002]. The investigators concluded that the cerebellar hypoplasia reported in infancy in most children with CDG-Ia likely reflects atrophy of antenatal onset, rather than hypoplasia.
More extensive testing for CDGs has led to the identification of milder CDG-Ia phenotypes [Briones et al., 2002; de Lonlay et al., 2001; Grünewald, 2009]. The patients often have high residual levels of PMM2 activity [Drouin-Garraud et al., 2001; Grünewald et al., 2001; Westphal et al., 2001b]. Some patients have only borderline cognitive impairment, but strabismus persists in these very mild cases. Few adult CDG-Ia patients are employed. A longitudinal study of eight Spanish patients confirmed the wide range of clinical manifestations, ranging from neonatal hemorrhage, non-immune hydrops, and death, through mental retardation and motor impairment without acute decompensation in patients in their 20s, to one individual with normal development and only gastrointestinal dysfunction in childhood [Perez-Duenas et al., 2009].
The carrier frequency of the most common mutant allele is about 1 in 70 in the northern European population [Schollen et al., 2000]. It is believed to be lethal in the homozygous state. Genotype-phenotype correlations have not been informative, but some evidence suggests that frequent polymorphisms in other glycosylation-related genes may influence the severity of the phenotype. No effective specific therapy for CDG-Ia exists. Experiments using CDG-Ia cells suggested that increasing dietary mannose might improve glycosylation in patients, but clinical trials demonstrated no benefit [Kjaergaard et al., 1998; Marquardt et al., 1997; Mayatepek et al., 1997].
Population studies find the risk of having a second child with CDG-Ia to be close to 1 in 3, rather than the expected mendelian ratio of 1 in 4, suggesting that reduced glycosylation may have some selective advantage [Schollen et al., 2004b]. At-risk couples should be counseled appropriately.
MPI-CDG (Ib)
MPI-CDG (Ib) is caused by mutations in MPI, the gene encoding phosphomannose isomerase, which interconverts Man-6-P and Fructose (Frc)-6-P. This reaction produces most of the mannose for glycoprotein synthesis [de Koning et al., 1998; Niehues et al., 1998]. About 25 patients have been identified since its discovery in 1998 [de Lonlay and Seta, 2009]. This phenotype is not associated with any primary neurologic symptoms. Gastrointestinal and hepatic pathology, with hypoglycemia, coagulopathy, and protein-losing enteropathy, is characteristic.
MPI-CDG (Ib) is unique in that simple dietary mannose therapy corrects the abnormalities, except for liver fibrosis [de Lonlay and Seta, 2009; Durand et al., 2003; Niehues et al., 1998]. The dietary mannose is taken up through transporters and converted to Man-6-P, thereby bypassing the metabolic block. Mannose has no known side effects at the concentrations used. Nonenzymatic protein glycation occurs at high concentrations of mannose, which is considerably more reactive than glucose, and can raise hemoglobin A1c (HbA1c) levels [Harms et al., 2002]. Some patients have received such treatment for longer than 10 years without complications [Westphal et al., 2001a; de Lonlay and Seta, 2009]. Twenty percent of the patients who were subsequently confirmed to have phosphomannose isomerase deficiency, however, died before discovery of the disorder [Freeze, 2001a]. The potentially fatal outcome and availability of simple, effective treatment mandate investigation for CDGs in suspected cases. One adult patient with MPI deficiency was not able to tolerate oral mannose, but her protein-losing enteropathy appeared to respond to heparin therapy [Liem et al., 2008].
ALG6-CDG (Ic)
ALG6-CDG (Ic) resembles, but is less severe than PMM2-CDG. It is characterized by moderate psychomotor retardation, hypotonia, esotropia, seizures, and ataxia. Nevertheless, at least five children have died of CDG-related complications [Marquardt and Denecke, 2003; Newell et al., 2003; Westphal et al., 2000]. The defect is in a glycosyltransferase, hALG6, that results in production of a truncated lipid-linked oligosaccharide sugar chain, which is inefficiently transferred to proteins. Patients sometimes experience life-threatening protein-losing enteropathy during bouts of gastroenteritis [Westphal et al., 2000]. Skeletal dysplasia, including a unique form associated with brachytelephalangy has been described in a compound heterozygote for ALG6 [Drijvers et al., 2010]. An adult woman has been identified in whom ALG6 deficiency was associated with mental retardation, skeletal anomalies, virilization, and deep vein thrombosis [Sun et al., 2005]. ALG6 deficiency was first identified in 1998 and subsequently in more than 35 patients [Haeuptle and Hennet, 2009; Burda et al., 1998; Grünewald et al., 2000; Imbach et al., 2000a], making it the second most common form of CDG.
NOT56L-CDG (Id)
Patients with NOT56L-CDG have mutations in NOT56L (ALG3), which encodes the mannosyltransferase used to synthesize Man6GlcNAc2 [Körner et al., 1999]. The index patient had microcephaly, optic nerve atrophy, iris colobomas, epilepsy, spastic quadriparesis, and profound psychomotor delay. Another patient had similar features plus Dandy–Walker malformation, with agenesis of the cerebellar vermis and corpus callosum. She also had recurrent hypoglycemia, thrombocytopenia, hypoalbuminemia, and coagulopathy. A total of eight children with NOT56L-CDG have now been described; all have a severe phenotype, with 7 of the 8 manifesting neurological impairments, including seizures, visual impairment, psychomotor retardation, and cerebral or cerebellar hypoplasia; there were varying combinations of hepatic, hematologic, endocrine, and dysmorphic manifestations, as well [Kranz et al., 2007b].
ALG12-CDG (Ig)
Eight cases of ALG12-CDG (Ig) have been reported [Chantret et al., 2002; Grubenmann et al., 2002; Thiel et al., 2002; Eklund et al., 2005; Kranz et al., 2007c]. The patients had typical CDG abnormalities, including hypotonia, generalized developmental delay, cerebellar hypoplasia, and decreased coagulation factors. Dysmorphic features included a long thin face, flat nasal bridge, and epicanthal folds. Frequent infections probably reflect reduced immunoglobulin G (IgG) concentrations. Affected males have genital hypoplasia, a feature not reported in other types of CDGs. More recent reports have emphasized features of skeletal dysplasia. These patients have mutations in hALG12, which encodes dolichol-P-mannose: Man7GlcNAc2-PP-dolichyl mannosyltransferase. Patients accumulate the truncated lipid-linked oligosaccharide, which is inefficiently transferred to proteins.
ALG8-CDG (Ih)
Eight patients have been described with ALG8-CDG (Ih), caused by a deficiency in hALG8, the gene encoding the second glucosyltransferase in lipid-linked oligosaccharide synthesis [Chantret et al., 2003; Schollen et al., 2004a; Stolting et al., 2009; Vesela et al., 2009]. Three patients had mild clinical presentations, including two siblings with pseudogynecomastia, epicanthus, hypotonia [Stolting et al., 2009], mental retardation, and ataxia, whereas the others all had severe multi-organ failure. Most died within a few months, but one patient with severe developmental delay survived a year before succumbing to renal failure. The others experienced hepatointestinal symptoms; one girl had cortical, cerebellar, and optic atrophy and intractable seizures before her death from systemic complications at 2 months [Vesela et al., 2009].
ALG2-CDG (Ii)
The only child with ALG2-CDG (Ii) recognized to date was normal at birth, except for an iris coloboma. Seizures, hepatomegaly, and coagulation abnormalities emerged in the first year. Imaging studies revealed cerebral hypomyelination. Pathologic mutations were found in hALG2, the enzyme that adds the second mannose to the growing lipid-linked oligosaccharide sugar chain [Thiel et al., 2003].
DPAGT1-CDG (Ij)
DPAGT1-CDG (Ij) is caused by a deficiency in UDP-GlcNAc:dolichol phosphate N-acetylglucosamine-1 phosphate transferase (GPT) activity encoded by DPAGT1. Two patients had severe hypotonia, intractable seizures, mental retardation, microcephaly, and exotropia [Wu et al., 2003].
ALG1/HMT1-CDG (Ik)
Ten children with HMT1-CDG (Ik) have been described [Kranz et al., 2004; Schwarz et al., 2004; Dupre et al., 2010]. Fifty percent had complications during pregnancy, and several had postnatal complications. Eighty percent were hypotonic, and all had at least one seizure, most being intractable; 8 of the 10 were dysmorphic, 7 of the 10 had visual impairment, and 5 of the 10 were microcephalic. Fifty percent had a fatal outcome. Patients with HMT1-CDG are deficient in GDP-Man:GlcNAc2-P-P-dolichol mannosyltransferase, encoded by the hALG1 gene, which adds the first Man to the lipid-linked oligosaccharide chain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree