Stroke type
Percentage (% of each category)
Ischemic
85
Carotid artery circulation
55
Vertebral/basilar artery circulation
12
Lacune
31
Other (vasculopathy, coagulopathy, sickle cell, hyperviscosity, vasculitis)
2
Hemorrhagic
14
Hypertensive hemorrhage
50
Amyloid angiopathy hemorrhage
15
Saccular aneurysm
30
Arteriovenous malformation
3
Other (infective aneurysm, cocaine, anticoagulants)
2
Venous
1
Thrombosis of cortical veins, deep cerebral veins, or dural sinuses
Stroke is the third leading cause of death in the USA. Each year 800,000 people in the USA develop stroke and 175,000 die. Fortunately, since 1960 the incidence of strokes in the USA has significantly fallen by 50 % primarily due to better control of hypertension, diabetes mellitus, and smoking but still 4 million adults in the USA are found to have stroke with an overall stroke prevalence of 750 per 100,000.
Ischemic Stroke
Introduction
Ischemic stroke occurs from lack of sufficient arterial blood flow in the territory of a specific cerebral artery to maintain neuronal viability. The stroke can be due to (1) intrinsic vascular occlusion (thrombus) that occurs in the neck portion of the internal carotid, vertebral artery, or a cerebral artery or (2) vascular occlusion with material originating elsewhere (embolism) such as a stenotic site of the internal carotid or vertebral artery or from the heart. The large majority of emboli are blood clots but occasionally they can be air, fat, or tissue fragments. Eighty percent of ischemic strokes involve the carotid artery territory or anterior circulation and 20 % involve the vertebrobasilar artery or posterior circulation.
The cause of a stroke depends on the patient’s age. In children, congenital heart disease with paradoxical embolism, Moyamoya disease, bacterial endocarditis, rheumatic fever, sickle cell anemia, and mitochondrial disorders should be considered. In young adults, estrogen-related stroke, migraine, vascular malformation , arteritis, hypercoagulation abnormalities, arterial dissections, and drug reactions from amphetamines, heroin, and cocaine may be seen. In middle age, hypertensive hemorrhage , ruptured saccular aneurysm , fibromuscular dysplasia and cardiogenic embolism often occur. The elderly often develop lacunar strokes , multi-infarct dementia , and atherosclerotic thrombotic strokes.
Table 9.2 lists the major modifiable and unmodifiable risk factors for stroke.
Table 9.2
Risk factors for stroke
Risk factor | Increase in risk over normal age-matched population |
|---|---|
Modifiable | |
Hypertension | 300–600 % |
Diabetes mellitus | 200–400 % |
Smoking | 150–300 % |
Cocaine/Crack | 200–500 % |
Atrial fibrillation Untreated On warfarin | 300–500 % 50 % |
Other heart abnormalities (mural thrombus, cardiomyopathy, acute myocardial infarction, mechanical heart valve, infective endocarditis) | 200–600 % |
Obesity | 200 % |
Serum lipid abnormalities | 50 % |
Asymptomatic carotid artery stenosis 60 to 74 % stenosis 75 to 94 % stenosis Greater than 95 % stenosis Total occlusion | (Five year increase) 10 % 14 % 10 % 5 % |
Unmodifiable | |
Advancing age | Relative rates double every decade after age 55 years |
Male gender | 25 % |
Prior transient ischemic attack | 400 % |
Heredity (first degree relative with stroke) | 100–400 % depending on cause |
Pathophysiology
Cerebral ischemia occurs from inadequate cerebral blood flow to the brain area. Total lack of oxygen and glucose to all brain neurons, as in a 12 to 15 s cardiac arrest, suppresses electrical activity and causes loss of consciousness. Normally cerebral arterial blood flow is 50 mL/100 g brain/minute. When cerebral blood flow falls below 18 mL/100 g brain/minute, cerebral function falters but neurons may remain alive (Fig. 9.1). Thus, electrical activity ceases and sodium/potassium pumps begin to fail but the neurons are viable and can recover function if blood flow improves. In a stroke , this area of potential recovery is called an ischemic penumbra . Blood flow below 8 mL/100 g brain/minute results in neuronal death as early as 15 min after flow disruption. Neurons in the hippocampus and cerebellum are most sensitive to ischemia while neurons in the brainstem and spinal cord are the most resistant. Brain ischemia results in impaired energy metabolism with accumulation of calcium ions in the intracellular space, elevated lactate levels, acidosis, and production of free radicals. Cellular homeostasis is disrupted leading to neuronal death.
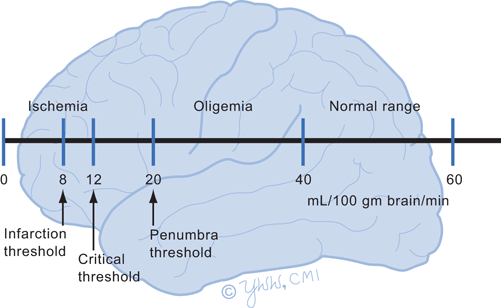

Fig. 9.1
Cerebral blood flow thresholds
Stroke from occlusion of a specific cerebral artery causes a wedge-shaped infarction (Figs. 9.2 and 9.3). If a large artery occludes, such as the middle cerebral artery, the stroke may involve that entire vascular territory or portions may be spared depending on the degree of collateral circulation. In about 25 % of ischemic strokes , there is rapid reperfusion of the ischemic territory from lysis of the embolic clot allowing blood to leak from damaged small arterioles, capillaries, and venules producing hemorrhagic transformation of varying degrees within the ischemic stroke.
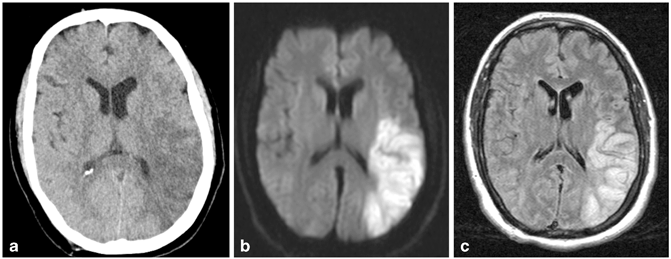
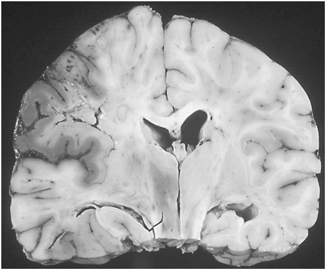

Fig. 9.2
Acute left middle cerebral artery wedge-shaped infarct shown by hypodensity on noncontrast head computed tomography (CT) (a), by bright signal on diffusion-weighted magnetic resonance imaging (MRI) (b) and by bright signal on T2 FLAIR MRI (c). (Courtesy of Blaine Hart, MD)

Fig. 9.3
Ischemic infarction, wedge-shaped pathologic specimen. (Courtesy of Mark Becher, MD)
Border zone or watershed infarctions are ischemic lesions that occur in characteristic locations at the junctions between two nonanastomosing arterial territories and constitute about 10 % of ischemic infarctions. Hemodynamically, they usually develop from severe prolonged hypoperfusion of the watershed territory resulting from some combination of systemic hypotension, tight stenosis or occlusion of extracranial or intracranial arteries, and microemboli that originate from the heart or the proximal stenotic artery lodging in distal intracranial arteries. The most common locations are between lenticulostriate and middle cerebral arteries and the middle and anterior cerebral arteries. The signs and symptoms produced are often mild, variable, and usually patients make a good recovery. Exceptions are watershed brainstem infarctions involving branches of the basilar artery.
Microscopically, a large vessel ischemic stroke shows little visible changes until about 6 h later when swelling of neurons, astrocytes, and endothelial cells begins. Neurons first swell, then shrink, develop chromatolysis (nuclei become eccentric with hyperchromasia) and then die. Neutrophils are abundant after the first day. By second day microglia proliferate and become macrophages engulfing myelin breakdown products. Astrocytes proliferate, become reactive, and lay down glial fibers to produce gliosis. Neovascularity slowly develops and renourishes the damaged brain. Gradually over months the necrotic brain products are reabsorbed producing a glial lined cavity of variable size, seen on neuroimaging as encephalomalacia.
Lacunar ischemic strokes represent 25 % of ischemic strokes and differ from large arterial strokes. They are small, less than 15 mm in diameter, and are primarily located in the basal ganglia, thalamus, brainstem, internal capsule, and centrum semiovale. Although many lacunes are silent producing with no symptoms, those that do cause clinical symptoms tend to be located at strategic sites where descending and ascending long tracts to and from the cortex are concentrated. Lacunes produce five well-recognized syndromes: pure motor hemiparesis (65 %), sensorimotor stroke (20 %), ataxic hemiparesis (9 %), pure sensory stroke (5 %), and dysarthria-clumsy-hand syndrome (1 %). Acute lacunes are best recognized as small round lesions on magnetic resonance imaging (MRI) diffusion-weighted images. Older lacunes appear as hypointense small round cystic circles on T1-weighted MRI and must be distinguished from benign similar appearing vessels surrounded by a dilated Virchow–Robin space.
Lacunes are caused by arteriole microvascular occlusion of a single deep perforating artery less than 200 μ in diameter. The arterial pathology shows arteriolosclerotic or lipohyalinosis changes characterized by a disorganized occluded vessel wall, which has been replaced by connective tissue and surrounding macrophages. The predisposing factors for lacunes are unclear but advancing age and hypertension are the best recognized. The prognosis for good recovery is better than for major artery ischemic strokes. The rate of developing subsequent lacunes is 5 % per year.
The mechanism of natural stroke recovery is incompletely understood. Possible mechanisms for motor recovery include: early recovery of motor neuronal excitability as blood flow increases; and later, (1) activation of partially spared corticospinal tract pathways; (2) alternate behavioral strategies to use limbs; (3) expansion or neuroplasticity of functional motor cortex within its existing normal domain; and (4) neuroplasticity of motor cortex within a new brain area. There is increasing evidence that the motor cortex is not fixed but plastic and can expand or shrink within the existing site based on clinical demand and can even move motor function to remote sites. However, movement of the motor cortex to a different gyrus likely occurs only in young children.
Major Clinical Features
Onset is sudden or the patient awakens from sleep with the completed stroke but rarely the stroke signs can progress over 1–2 days. Table 9.3 lists the common clinical features of lacunar, anterior circulation, and posterior circulation strokes while Fig. 9.4a, b show the location and distribution of the major arteries. Most cortical strokes are symptomatic but only one-third of lacunes are symptomatic. Overall in cortical ischemic strokes, the hemiparesis is severe in 60 %, moderate in 20 %, and mild in 20 %. Broca’s aphasia is more common than Wernicke’s aphasia but in large left middle cerebral artery strokes, a global aphasia will be present (see Chap. 11 on disorders of higher cognitive function).
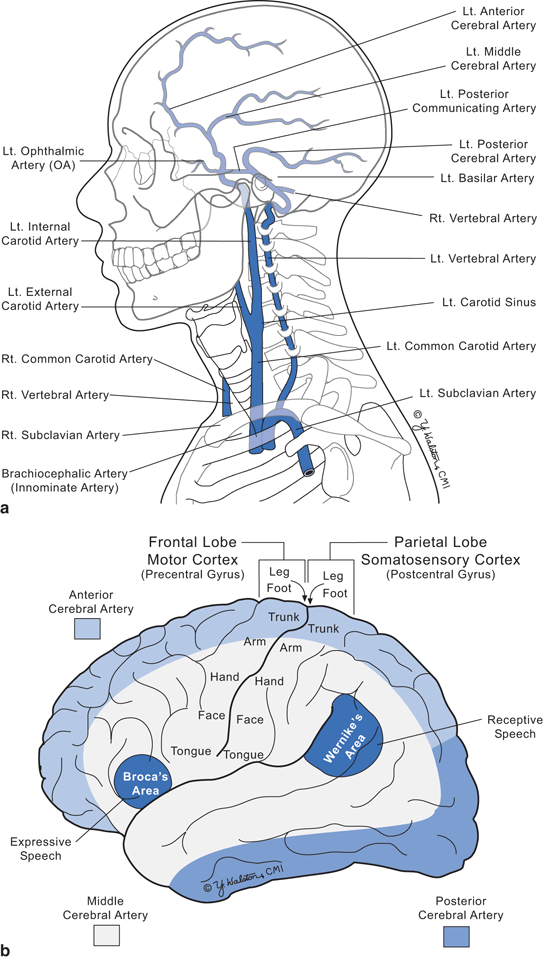

Fig. 9.4
Arterial supply of the brain. a Major vessels b Vascular territories
Table 9.3
Clinical features of common strokes
Arterial territory of stroke | Clinical presentationa |
|---|---|
Left middle cerebral artery (mid frontal and parietal lobes) | Aphasia, contralateral hemiparesis, contralateral hemisensory loss, homonymous hemianopia, dysphagia |
Right middle cerebral artery (mid frontal and parietal lobe) | Contralateral hemiparesis, contralateral hemisensory loss, homonymous hemianopia, dysphagia, apraxia |
Anterior cerebral artery (frontal pole and medial aspect of frontal and parietal lobes) | Contralateral leg weakness and sensory loss |
Vertebral/basilar artery | |
Wallenberg’s syndrome from posterior inferior cerebellar artery (medulla and cerebellum) | Vertigo, nystagmus, dysphagia and dysarthria with ipsilateral Horner’s (miosis, ptosis, and diminished sweating on face) diminished facial pain and temperature perception, limb ataxia and contralateral loss of trunk and limb pain and temperature |
Mid basilar artery (pons and cerebellum) | Often involves bilateral branches producing signs that include facial weakness, quadraparesis, dysarthria, dysphagia, vertical and horizontal nystagmus, ptosis, skew deviation of vision, limb ataxia, and diminished level of consciousness Locked-in syndrome occasionally develops with complete loss of voluntary limb and face movement, retained consciousness and voluntary vertical eye movements |
Top of basilar artery (midbrain, occipital lobes and temporal lobes) | Involves midbrain and posterior cerebral arteries producing disruption of voluntary vertical gaze, third nerve palsies, ataxia, somnolence, homonymous hemianopia or quadrantopia, and occasionally loss of recent memory |
Lacunar stroke territory | |
Internal capsule | Contralateral hemiparesis, hemisensory loss without aphasia or visual loss |
Upper half brainstem/cerebellum | Combinations of ataxia, vertigo, diplopia, dysarthria, Horner’s sign, contralateral sensory loss, ipsilateral facial weakness, ipsilateral facial sensory loss |
Lower half brainstem | Contralateral hemiparesis without sensory loss (pure motor stroke) |
Major Laboratory Findings
Tests are performed to diagnose a stroke, identify its location and determine the cause and source. Computed tomography (CT) scans are excellent to detect a hemorrhagic stroke but often appear normal for 6 to 24 h following an acute ischemic stroke. Subtle effacement (loss of boundaries) of sulci is the earliest sign followed by development of a hypodense region due to development of cytotoxic and vasogenic edema . In general, the larger the stroke the earlier it becomes visible on neuroimaging. MRI is the most sensitive neuroimaging method to detect an ischemic stroke. While conventional MRI may appear normal for several hours, diffusion-weighted MRI will show an area of hyperintensity in the territory of the infarct within 4 h. Diffusion-weighted MRI scans are helpful to distinguish an acute stroke from older strokes that are not hyperintense. Within 8 h, edema from the infarction appears hyperintense on T2-weighted images and hypointense on T1-weighted images. MRI is sensitive for small lacunes and infarctions in the brainstem and cerebellum that may be missed by CT. In a patient with a lacunar stroke it is common to identify other older lacunes that were clinically silent.
Several tests are used to determine the cause of the stroke. Imaging of the extra- and intracranial vessels is done with either magnetic resonance angiography (MRA) or CT angiography (CTA) and can identify medium to large diameter stenotic or occluded arteries in the neck and head. Extracranial Doppler ultrasonography examination of the carotid artery in the neck also detects narrow or occluded vessels. Transthoracic or transesophageal echocardiogram can detect clots or masses within the heart, vegetations on heart valves, immobile cardiac wall segments, and cardiomegaly that point to a cardioembolic source. Cerebrospinal fluid (CSF) examination is seldom necessary but abnormal CSF can indicate a vasculitis or meningitis and CSF culture may determine the cause of the meningitis. A variety of blood tests can look for a coagulopathy or vasculopathy (see transient ischemia attack section for details).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








