Drugs and other physical treatments
History of physical treatments
The classification of drugs used in psychiatry
Introduction
This chapter is concerned with the use of drugs and other physical treatments, such as electroconvulsive therapy and neurosurgical procedures. Psychological treatments are the subject of Chapter 20. This separation, although convenient when treatments are described, does not imply that the two kinds of therapy are to be thought of as exclusive alternatives when an individual patient is considered; on the contrary, many patients require both. In this book, the ways of combining treatments are considered in other chapters where the treatment of individual syndromes is discussed. It is important to keep this point in mind when reading this chapter and the next.
Our concern is with clinical therapeutics rather than basic psychopharmacology, which the reader is assumed to have studied already. An adequate knowledge of the mechanisms of drug action is essential if drugs are to be used in a rational way, but a word of caution is appropriate. The clinician should not assume that the therapeutic effects of psychotropic drugs are necessarily explained by the pharmacological actions that have been discovered so far. In addition, there is relatively little knowledge about the neuropsychological mechanisms through which pharmacological manipulation can ameliorate psychological symptomatology.
This caution does not imply that a knowledge of pharmacological mechanisms has no bearing on psychiatric therapeutics. On the contrary, there have been substantial advances in pharmacological knowledge since the first psychotropic drugs were introduced in the 1950s, and it is increasingly important for the clinician to relate this knowledge to their use of drugs. As noted in Chapter 6, evidence-based clinical guidelines are increasingly used to assist practitioners in their use of medication, and it is important to be aware of these. However, in specialist psychiatric practice there is often rather little high-quality evidence to guide prescribing, and a knowledge of pharmacology will help to ensure that safe and reasonable prescribing practices are followed.
History of physical treatments
Physical treatments have been applied to patients with psychiatric disorders since antiquity, although, in retrospect, the most that could be claimed for the best of these interventions is that they were relatively harmless. Of course, the same holds for the management of patients with general medical disorders, for which similar treatments, such as bleeding and purging, were often used regardless of diagnosis. It is wise not to be too censorious about the treatment of disorders of which the aetiology is still largely unknown, but to bear in mind that ‘it may well be that in a hundred years current therapies, psychotherapies as well as physical therapies, will be looked upon as similarly uncouth and improbable’ (Kiloh et al., 1988).
Historically, physical treatments can be divided into two main classes:
• those that were aimed at producing a direct change in a pathophysiological process, usually by some alteration in brain function
• those that were aimed at producing symptomatic improvement through a dramatic psychological impact.
The latter interventions were often based on philosophical theories about the moral basis of madness. For example, many physicians appear to have followed the proposal of Heinroth (1773–1843) that insanity was the product of evil and personal wrongdoing. Accordingly, restraint with chains and corporal punishment were seen as appropriate remedies. Other physical treatments, such as the spinning chair introduced by Erasmus Darwin (1731–1802), seemed to be designed to produce a general ‘shock to the system’, and perhaps thereby to interrupt the morbid preoccupations of the patient. A less arduous regimen was the use of continuous warm baths, often given in combination with cold packs. This treatment was recommended by clinicians as distinguished as John Connolly (1794–1866) and Emil Kraepelin (1856–1926), and was still in use at the Bethlem Hospital in the 1950s.
Drugs that produce changes in the function of the central nervous system, such as opiates and anticholinergic agents, have been used in the treatment of mental disorders for hundreds of years. Although some of these drugs may sometimes have had calming effects, they were of no specific value in the treatment of psychiatric disorders. Often a physical treatment was used not because of its proven efficacy, but because it was recommended by an eminent and vigorous physician. Also, the assessment of efficacy depended almost entirely on uncontrolled clinical observation.
In 1933, about 10 years after the isolation of insulin by Frederick Banting and Charles Best, Manfred Sakel introduced insulin coma treatment for psychosis (Sakel, 1938). A suitable dose of insulin was used to produce a coma, which was terminated by either tube feeding or intravenous glucose. A course of treatment could include up to 60 comas. Not surprisingly, serious side-effects were common, and a mortality of at least 1% could be expected, depending on the standard of the clinic and the physical state of the patient. Insulin coma treatment was rapidly taken up throughout Europe, and many specialized treatment units were built. There was a great improvement in the morale of patients and staff because of the belief that this dramatic treatment could cure symptoms of some of the most serious psychiatric disorders.
There were always some practitioners who doubted the efficacy of insulin coma treatment. Their doubts were reinforced by a controlled trial by Ackner and Oldham (1962), who found that, in patients with schizophrenia, insulin coma was no more effective than a similar period of unconsciousness induced by barbiturates. This study was published about the time when chlorpromazine was introduced, and both factors led to a rapid decline in the use of insulin coma treatment. It should be noted that the controlled studies did not exclude the efficacy of insulin treatment in some circumstances, and a number of workers continued to maintain that it was effective. Therefore it is interesting that recent experimental studies have shown that insulin administration causes striking changes in the release of monoamine neurotransmitters in the brain. Perhaps the main lesson to be learned from insulin coma treatment is that the introduction of a new medical treatment should be preceded by adequate controlled trials to determine whether it is therapeutically more effective or safer than current therapies (see Chapter 6). This lesson is particularly important in psychiatry, because the aetiology of most disorders is obscure and outcome may vary widely, even among patients with the same clinical syndrome.
Electroconvulsive therapy (ECT) was introduced about the same time as insulin coma treatment. Unlike the latter, ECT has retained a place in current clinical practice. The rationale for convulsive therapy was a postulated antagonism between schizophrenia and convulsions such that the one would exclude the other. This view is erroneous in so far as schizophrenia-like illnesses are actually more common in patients with temporal lobe epilepsy than would be expected by chance (see p. 339). Astute clinical observation, in combination with controlled trials, has shown that ECT is effective in the acute treatment of severe mood disorders. Thus, even though the rationale for the introduction of ECT was incorrect and its mode of action remains unclear, controlled trials have confirmed that, in carefully defined clinical situations, ECT is a safe and effective treatment (see p. 557).
The action of lithium in reducing mania was a chance finding by Cade (1949), who had been investigating the effects of urates in animals and had decided to use the lithium salt because of its solubility. Lithium is a toxic agent, so Cade’s important observations did not have a significant impact on clinical practice until the following decade, when controlled trials showed that lithium was effective in both the acute treatment of mania and the prophylaxis of recurrent mood disorders.
Other agents that revolutionized psychopharmacology were introduced about this time (see Box 19.1). Their efficacy and their indications were first recognized through clinical observation, and were subsequently confirmed by controlled clinical trials. None of these agents was introduced on the basis of an aetiological hypothesis. Indeed, such aetiological hypotheses as there are in biological psychiatry have been largely derived from knowledge of the mode of action of effective drugs. Thus the dopamine-receptor-antagonist properties of antipsychotic drugs have given rise to the dopamine hypothesis of schizophrenia, while the action of tricyclic antidepressants and monoamine oxidase inhibitors (MAOIs) in facilitating the effects of noradrenaline and 5-hydroxytryptamine (5-HT) has led to the various monoamine hypotheses of mood disorders.
The last 30 years have brought a period of consolidation in psychopharmacology. Clinical trials have been widely used to refine the indications of particular drug treatments and to maximize their risk/benefit ratios. New compounds have become available, but because most of them have been derived from previously described agents, their range of activity is not strikingly different from that of their predecessors. In general, however, the newer agents are better tolerated and sometimes safer—developments which are important for clinical practice.
There may now be grounds for more optimism about the prospects for advances in psychopharmacology. For example, there is rapidly increasing knowledge about chemical signalling in the brain. Numerous neurotransmitters and neuromodulators interact with specific families of receptors, many of which exist in a number of different subtypes. Most of these receptors have been cloned, and selective ligands for them are becoming available. There is increasing knowledge as to how these chemical messengers may modify behaviour through their interactions with specific brain regions and distributed neuronal circuits.
Future compounds that are developed as consequence of these scientific advances are likely to differ from current drugs in their range of behavioural effects, and could lead to important new developments in psychopharmacology. Given the complex causes of psychiatric disorders, it seems likely that detailed knowledge of aetiology and pathophysiology may lag behind advances in therapeutics. Of course, this disparity is not uncommon in general medicine. It serves to reinforce the importance of randomized clinical trials in the assessment of new psychopharmacological treatments.
General considerations
The pharmacokinetics of psychotropic drugs
Before psychotropic drugs can produce their therapeutic effects, they must reach the brain in adequate amounts. The extent to which they do so depends on their absorption, metabolism, excretion, and passage across the blood–brain barrier. A short review of these processes is given here. The reader who has not studied them before is referred to the chapter on pharmacokinetics in Grahame-Smith and Aronson (2002). The following processes are important:
• absorption
• distribution
• excretion.
Absorption
In general, psychotropic drugs are easily absorbed from the gut because most of them are lipophilic and are not highly ionized at physiological pH values. Like other drugs, they are absorbed faster from an empty stomach, and in reduced amounts by patients suffering from intestinal hurry or malabsorption syndrome.
Distribution
Psychotropic drugs are distributed in the plasma, where most of them are largely bound to proteins—for example, diazepam and amitriptyline are about 95% bound. They pass easily from the plasma to the brain because they are highly lipophilic. For the same reason, they enter fat stores, from which they are released slowly long after the patient has ceased to take the drug. This means that psychotropic drugs tend to have large volumes of distribution.
Metabolism
Most psychotropic drugs are metabolized in the liver. This process begins as the drugs pass through the liver in the portal circulation on their way from the gut. This ‘first-pass’ metabolism reduces the amount of available drug, and is one of the reasons why larger doses are needed when a drug such as chlorpromazine is given by mouth than when it is given intramuscularly. The extent of this liver metabolism differs from one person to another. It is altered by certain other drugs which, if taken at the same time, induce liver enzymes (e.g. carbamazepine) or inhibit them (e.g. selective serotonin reuptake inhibitors [SSRIs]).
Some drugs, such as carbamazepine, induce their own metabolism, especially after being taken for a long time. Not all drug metabolites are inactive—for example, fluoxetine is metabolized to a hydroxy derivative, norfluoxetine, which is also a potent 5-HT reuptake inhibitor. Where drugs give rise to active metabolites, measurements of plasma concentrations of the parent drug alone are a poor guide to therapeutic activity.
Excretion
Psychotropic drugs and their metabolites are excreted mainly through the kidney. When kidney function is impaired, excretion is reduced and a lower dose of drug should be given. Lithium is filtered passively and then partly reabsorbed by the same mechanism that absorbs sodium. The two ions compete for this mechanism; hence reabsorption of lithium increases when that of sodium is reduced (e.g. by thiazide diuretics). Certain fractions of lipophilic drugs such as chlorpromazine are partly excreted in the bile, enter the intestine for the second time, and are then partly reabsorbed (i.e. a proportion of the drug is recycled between intestine and liver).
Measurement of circulating drug concentrations
As a result of individual variations in the mechanisms described above, plasma concentrations after standard doses of psychotropic drugs vary substantially from one patient to another. For example, tenfold differences have been observed with the antidepressant drug nortriptyline. Therefore it might be expected that measurements of the plasma concentration of circulating drugs would help the clinician. However, with a few exceptions (e.g. lithium and clozapine), this practice is rarely helpful because the plasma drug levels that predict therapeutic response or toxicity within individuals vary so much. Clearly for any drug to work it must be present in plasma above a certain minimum concentration, and for some medications ‘target’ levels have been suggested. However, it is still not unusual for some individuals to show a therapeutic response when plasma levels are lower than recommended.
Pharmacodynamic measures
As an alternative to these assays, it may be possible to measure the pharmacological property which is thought to be responsible for the therapeutic effect of a particular drug. For example, positron emission tomography can be used to measure directly the degree of brain dopamine-receptor blockade produced by antipsychotic drugs during treatment. Such information has proved valuable in designing appropriate dosage regimens of antipsychotic drugs (see Table 19.6). However, these pharmacodynamic measures have not yet been able to identify why some patients do not respond to medication. For example, the degree of dopamine-receptor blockade is the same in patients who respond to antipsychotic drugs as in those who do not. Pharmacogenetic approaches to prediction of treatment response (see Box 19.2) are a topic of current interest, although most of the findings remain controversial and yet to have an impact on routine clinical practice. For a review, see Zandi and Judy (2010).
Plasma half-life
Plasma concentrations of drugs vary throughout the day, rising immediately after the dose and falling at a rate that differs between individual drugs and individual people. The rate at which a drug level declines after a single dose ranges from hours for lithium carbonate to weeks for slow-release preparations of injectable antipsychotic agents. Knowledge of these differences allows more rational decisions to be made about appropriate intervals between doses.
The concept of plasma half-life is useful here. The half-life of a drug in plasma is the time taken for its concentration to fall by a half, once dosing has ceased. For most psychotropic drugs, the amount eliminated over time is proportional to the plasma concentration, and in this case it will take approximately five times the half-life for the drug to be eliminated from plasma. Equally, when dosing with a drug begins, it will take five times the half-life for the concentration in plasma to reach steady state. This can be important when planning treatment. For example, MAOIs should not be given with SSRIs. Therefore if a patient is taking sertraline, which has an elimination half-life of about 26 hours, it will be important to leave at least five times the half-life (a week is recommended) before starting MAOI treatment. When sertra-line treatment begins, the plasma concentrations will continue to rise for about a week before reaching a steady state.
Drug interactions
When two psychotropic drugs are given together, one may interfere with or enhance the actions of the other. Interference may arise through alterations in absorption, binding, metabolism, or excretion (pharmacokinetic interactions), or by interaction between the pharmacological mechanisms of action (pharmacodynamic interactions).
Pharmacokinetic interactions
Interactions that affect drug absorption are seldom important for psychotropic drugs, although it is worth noting that absorption of chlorpromazine is reduced by antacids. Interactions due to protein binding are also uncommon, even though many psychotropic drugs are highly protein bound. Interactions that affect drug metabolism are of considerable importance. Examples include the inhibition of the metabolism of antipsychotic drugs by SSRIs, and the stimulation of the metabolism of many psychotropic drugs by carbamazepine, which induces the relevant cytochrome P450 enzymes (see below). Interactions that affect renal excretion are mainly important for lithium, the elimination of which is decreased by thiazide diuretics.
Cytochrome P450 enzymes. There have been significant developments in the understanding of the microsomal cytochrome P450 enzyme system. These enzymes are located mainly in the liver but also in other tissues, including the gut wall and brain. Their role is to detoxify exogenous substances such as drugs, and their activity can be increased or decreased by concomitant drug administration. This can give rise to clinically important drug interactions (Grahame-Smith and Aronson, 2002). Importantly, several new antidepressants, particularly selective SSRIs, potently inhibit P450 enzymes (see Table 19.11).
Pharmacodynamic interactions
Pharmacodynamic interactions are exemplified by the serotonin syndrome, in which drugs that potentiate brain 5-HT function by different mechanisms (e.g. SSRIs and MAOIs) can combine to produce dangerous 5-HT toxicity (see p. 541).
As a rule, a single drug can be used to produce all of the effects required of a combination—for example, many antidepressant drugs have useful anti-anxiety effects. It is desirable to avoid combinations of psychotropic drugs whenever possible, and if a combination is to be used, it is essential to know about possible interactions. The British National Formulary provides a useful guide.
Drug withdrawal
Many psychotropic drugs do not achieve useful therapeutic effects for several days or even weeks. After the drugs have been stopped, there is often a comparable delay before their effects are lost. Psychotropic and indeed many other classes of drugs produce neuroadaptive changes during repeated administration. Tissues therefore have to readjust when drug treatment is stopped; this readjustment may appear clinically as a withdrawal or abstinence syndrome. Characteristic abstinence syndromes have been described for antidepressants, antipsychotics, and anxiolytics, while sudden discontinuation of lithium can provoke a ‘rebound’ mania. It is important to be able to distinguish withdrawal syndromes from relapse of the disorder that is being treated. In addition, the risk of abstinence symptoms makes it prudent to withdraw psychotropic drugs slowly wherever possible.
General advice about prescribing
Use well-tried drugs
It is good practice to use well-tried drugs with therapeutic actions and side-effects that are thoroughly understood. The clinician should become familiar with a small number of drugs from each of the main classes. In this way he can become used to adjusting the dosage and recognizing side-effects. Well-tried drugs are usually less expensive than new preparations.
Give an adequate dose
Having chosen a suitable drug, the doctor should prescribe it in adequate doses. He should not change the drug or add others without a good reason. In general, if there is no therapeutic response to one established drug, there is no likelihood of a better response to another that has very similar pharmacological properties (provided that the first drug has been taken in adequate amounts). However, since the main obstacle to adequate dosage is usually side-effects, it may be appropriate to change to a drug with a different pattern of side-effects—for example, from a tricyclic antidepressant to an SSRI, or vice versa.
Use drug combinations cautiously
Occasionally, combinations of psychotropic drugs are given deliberately in the hope of producing interactions that will be more potent than the effects of either drug taken alone in full dosage (e.g. a tricyclic antidepressant with a MAOI). This practice, if it is to be used, is best carried out by experienced psychiatrists (or under their guidance), because the adverse effects of combinations are less easy to predict than those of single drugs.
Dosing and treatment duration
When a drug is prescribed, it is necessary to determine the dose, the interval between doses, and the likely duration of treatment. The dose ranges for commonly used drugs are indicated later in this chapter. Ranges for others can be found in the manufacturers’ literature, the British National Formulary, or a comparable work of reference. Within the therapeutic range, the correct dose for an individual patient should be decided after considering the severity of symptoms, the patient’s age and weight, and any factors that may affect drug metabolism (e.g. other drugs that are being taken, or renal disease).
Next, the interval between doses must be decided. Psychotropic drugs have often been given three times a day, even though their duration of action is such that most can be taken once or twice a day without any undesirable fall in plasma concentrations between doses. Less frequent administration has the advantage that outpatients are more likely to be reliable in taking drugs. In hospital, less frequent drug rounds mean that nurses have more time for psychological aspects of treatment. Some drugs, such as anxiolytics, are required for immediate effect rather than continuous action; they should not be given at regular intervals, but shortly before occasions on which symptoms are expected to be at their worst. The duration of treatment depends on the disorder under treatment; it is considered in the chapter that deals with the relevant clinical syndrome.
What patients want to know
Psychotropic drugs have the aim of changing what people think and feel; not surprisingly, many patients have misgivings about taking them. It is therefore important to make it clear what the drug is being used for, what therapeutic effects are expected, and when they should start to appear. Other key questions that must be dealt with include the following:
• What effects will I experience on first taking the drug?
• What side-effects can I expect?
• What serious side-effects should I report immediately?
• For how long should I take the drug?
• Is the drug addictive?
• What will I notice when I stop the drug?
Compliance, concordance, and collaboration
Many patients do not take the drugs that are prescribed for them. Of course this problem is not restricted to psychiatric practice, but the use of psychotropic drugs raises additional issues in terms of societal stigma and the nature of the adverse effects. Problems with compliance are mainly manifested when treating outpatients, but also occur in hospital, where some patients find ways of avoiding taking the drugs administered by nurses.
If patients are to take medication reliably, they must be convinced of the need to take it, be free from unfounded fears about its dangers, and be aware of how to take it. Each of these requirements presents particular problems when the patient has a psychiatric disorder. Thus patients with schizophrenia or seriously depressed patients may not be convinced that they are ill, or they may not wish to recover. Deluded patients may distrust clinical staff, and hypochondriacal patients may fear dangerous side-effects. Anxious patients often forget the prescribed dosage and frequency of their drugs. Therefore it is not surprising that many psychiatric patients do not take their drugs in the prescribed way. It is important for the clinician to pay attention to this problem. Time spent discussing the patient’s concerns is time well spent, for it often increases compliance with treatment. Written instructions can be a valuable adjunct, and are now often included with drug packaging.
The successful and safe use of medication requires an essentially collaborative relationship between patient and doctor. Some have proposed that the terms concordance or adherence should therefore be preferred to compliance, which carries the implicit assumption that the patient’s job is to obey instructions. Whatever the term used, it is clearly important to recognize that the use of drug treatment, particularly in psychiatry, requires a thorough understanding of the patient’s attitude to both their illness and its treatment (Chaplin et al., 2007; National Institute for Health and Clinical Excellence, 2009a; Britten et al., 2010).
Ethical aspects of drug prescription
The ethical issues in this complex area have been reviewed by Kader and Pantelis (2009).
1. The basis of ethical prescribing is the practitioner’s comprehensive knowledge of the risks and benefits of drug therapies. This will be derived from evidence-based approaches where possible.
2. The doctor–patient relationship is the appropriate framework through which this knowledge is communicated to the patient.
3. The therapeutic partnership between patient and doctor must lead to true informed consent, which includes the right of competent patients to refuse treatment.
Difficulties arise when the evidence base for treatment is lacking and when there is uncertainty about what approach to pursue. Here the clinician has the responsibility to advise treatments that would be supported by peer opinion and to use clinical guidelines. The clinician should also support the right of patients to genuinely effective treatment where this is being hindered by cost constraint and other economic factors. Another difficult problem concerns refusal of treatment when capacity is impaired and the health and safety of the patient or others are at risk. In fact, empirical research suggests that refusal of treatment in these circumstances is often transient and due to current clinical factors. Indeed most patients whose refusal of treatment is clinically overridden apparently conclude eventually that the decision to treat them was justified (Kader and Pantelis, 2009). It is, of course, important to elucidate and document the reasons for treatment refusal, and to respect the right of competent patients to refuse treatment.
Prescribing for special groups
Children and the elderly
Psychotropic drugs usually lack specific licences for use in young people, and relevant controlled trials are sparse. However, advice from the UK Committee on Safety of Medicines is that the efficacy of some SSRIs and venlafaxine treatment in depressed adolescents does not outweigh their disadvantages in terms of agitation and suicidal behaviour (see also Whittington et al., 2004). Practitioners need to make themselves aware of local guidelines concerning the use of psychotropic medication in young people, and should seek specialist advice when in doubt.
Clinical trials of most psychotropic medications often exclude older patients, even though conditions such as depression are more common in the elderly. Elderly patients are often sensitive to side-effects of medication, and may have impaired renal or hepatic function; for these reasons it is important to start treatment with low doses.
Pregnant women
There are special problems with regard to prescribing psychotropic drugs in pregnancy, because of the risk of teratogenesis. Information about the teratogenic risk of individual drugs can be obtained from the relevant manufacturer and from the British National Formulary, although the available evidence is often sparse or difficult to interpret. The practitioner and patient have the difficult task of weighing this information against the risk of managing the illness without medication. In addition, a substantial number of pregnancies are unplanned. For this reason, it is prudent where possible to advise women of childbearing age who require psychotropic drugs specifically to avoid pregnancy until the need for the drug treatment is over. For reviews of the use of psychotropic drugs in pregnancy, see National Institute for Health and Clinical Excellence (2007e) and Paton (2008).
Anxiolytics and antidepressants
Anxiolytic drugs are seldom essential in early pregnancy, and psychological treatments can usually be used. If medication is needed, benzodiazepines have in general not been shown to be teratogenic, although one meta-analysis has shown an increased risk of oral clefts after first-trimester exposure. If an antidepressant drug is required, it is probably better to use long-established preparations such as imipramine or amitriptyline for which there is no consistent evidence of a teratogenic effect after many years of use. There is also reasonable experience with fluoxetine, which does not appear to be associated with an increased risk of major malformations. However, paroxetine treatment in the first trimester may result in increased cardiac defects, and SSRIs taken after 20 weeks’ gestation may result in an increased risk of pulmonary hypertension in the newborn.
Antipsychotic drugs and mood stabilizers
It is seldom necessary to start antipsychotic drugs in early pregnancy. There is little evidence that high-potency agents such as haloperidol carry an increased teratogenic risk. However, there may be a higher rate of congenital malformations in babies who are exposed to lower-potency agents such as chlorpromazine. There is little information on the teratogenic risk of newer antipsychotic agents.
Lithium treatment early in pregnancy has been associated with cardiac abnormalities in the fetus, particularly Ebstein’s anomaly. Therefore women who are considering pregnancy have been recommended to discontinue lithium before conceiving. Similarly, women who become pregnant while taking lithium have usually been advised to stop the treatment. However, recent epidemiological studies have suggested that although the relative risk of Ebstein’s anomaly is increased at least tenfold in infants who are exposed to lithium in the first trimester, the absolute risk is still fairly low, at 0.05–0.1%. Withdrawal of lithium carries a high risk of relapse in patients with bipolar illness, and the balance of risk to mother and baby may therefore suggest continuation of lithium treatment during pregnancy in some cases.
Anticonvulsant drugs such as carbamazepine and valproate are increasingly used as mood stabilizers. However, both of these agents are clearly associated with an increased risk of neural-tube defects as well as other fetal abnormalities. The neural-tube defects associated with anti-convulsant use may be associated with changes in folate metabolism. However, a role for folate treatment in their prevention has not been established.
Neonatal toxicity
Exposure to psychotropic drugs in the later stages of pregnancy can give rise to neonatal toxicity either through the presence of the drug or through a withdrawal syndrome. For example, it has been reported that among babies born to mothers who have been receiving tricyclic antidepressants there may be withdrawal reactions that include tremulousness, vomiting, poor feeding, and seizures. Direct anticholinergic effects such as gastrointestinal stasis and bladder distension have also been reported. These reactions, although clearly problematic, appear to settle quickly without causing lasting sequelae.
There are also reports that late exposure to SSRIs may be associated with an increased risk of neonatal complications, including jitteriness, hypoglycaemia, poor muscle tone, and respiratory difficulties. The perinatal toxicity associated with lithium use includes ‘floppy baby syndrome’, with cyanosis and hypotonicity, whereas benzodiazepine treatment can result in impaired temperature regulation together with breathing and feeding difficulties.
Animal studies suggest that fetal exposure to psychotropic medication can cause longer-term abnormalities in brain development and behaviour, and it is possible that similar effects might occur in humans (see, for example, Pederson et al., 2010). However, disentangling such effects from those of depression is difficult (Grote et al., 2010).
Breastfeeding
Psychotropic drugs should be prescribed cautiously to women who are breastfeeding. Diazepam and other benzodiazepines pass readily into breast milk, and may cause sedation and hypotonicity in the infant. Antipsychotic drugs and antidepressants also enter breast milk, although rather less readily than diazepam. However, sulpiride is excreted in significant amounts and should be avoided. Fluoxetine and citalopram could also accumulate, but imipramine, nortriptyline, and sertraline are present in small amounts and breastfeeding can be permitted while the baby is observed for sedation or feeding difficulties. It is usually advised that mothers should express and discard breast milk that has been exposed to peak plasma levels of the drug concerned.
Lithium salts enter the milk freely, and serum concentrations in the infant can approach those of the mother, so breastfeeding requires great caution. However, the amounts of carbamazepine and valproate in breast milk are considered too low to be harmful. A general problem is that, even when the concentration of a particular drug in breast milk is low and no detectable clinical effect on the infant can be discerned, it is nevertheless possible that subtle longer-term effects on brain development and behaviour could occur. For this reason, some authorities recommend that women who are receiving psychotropic medication should not breastfeed at all. A more pragmatic view is provided by the British National Formulary (see also National Institute for Health and Clinical Excellence, 2007e; Moretti, 2009).
What to do if there is no therapeutic response
1. Is the drug being taken as recommended? The first step is to find out whether the patient has been taking the drug in the correct dose. They may not have understood the original instructions, or may be worried that a full dose will produce unpleasant side-effects. Some patients fear that they will become dependent if they take the drug regularly. Other patients may have little wish to take drugs for the reasons discussed above.
2. Is the patient is taking any other drug which could affect the metabolism or pharmacological action of the psychotropic agent? Misuse of legal or illegal substances might interfere with the therapeutic actions of psychotropic drugs.
3. Is the diagnosis correct? Review the diagnosis to make sure that the treatment is appropriate before deciding whether to increase the dose.
Failure to respond adequately to psychotropic medication is a common reason for psychiatric referral. Specific pharmacological approaches for individual disorders are discussed in the relevant chapters.
The classification of drugs used in psychiatry
Drugs that have effects mainly on mental symptoms are referred to as psychotropic. Psychiatrists also use the term antiparkinsonian agents, which refers to drugs that are employed to control the side-effects of some psychotropic drugs. Anticonvulsant drugs have a growing role in the treatment of mood disorders.
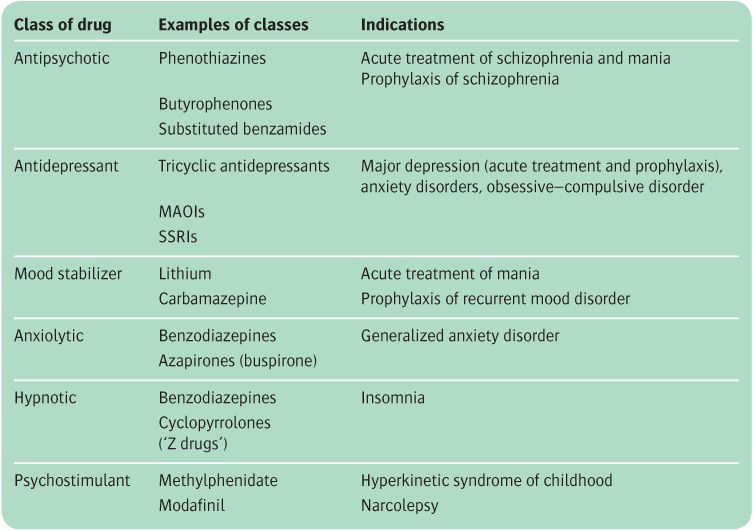
Psychotropic drugs are conventionally divided into different classes, as shown in Table 19.1, but the therapeutic actions of particular compounds are not confined to one diagnostic category. For example, SSRIs are classified as antidepressants and are effective in the treatment of major depression, but they also produce useful therapeutic effects in anxiety states, obsessive–compulsive disorders, and some eating disorders. Of course, this breadth of effect does not mean that the latter syndromes are forms of depression. It merely highlights the fact that the neuropsychological consequences of facilitating brain 5-HT function may provide beneficial effects in a variety of psychiatric disorders.
Although there is considerable understanding of the pharmacological actions of psychotropic drugs, little is known about the neuropsychological consequences of these pharmacological actions and about the ways in which neuropsychological changes are translated into clinical benefit in different diagnostic syndromes. At present, therefore, the best plan is to classify drugs according to their major therapeutic use, but to bear in mind that the therapeutic effects of different classes of drugs may overlap considerably.
Each of the main groups of drugs will now be reviewed in turn. For each group, an account will be given of therapeutic effects, pharmacology, the principal compounds available, pharmacokinetics, unwanted effects (both those appearing with ordinary doses and the toxic effects of unduly high doses), and contraindications. General advice will also be given about the use of each group in everyday clinical practice, but specific applications to the treatment of individual disorders will be found in the chapters that deal with those conditions. Drugs that have a limited use in the treatment of a single disorder—for example, disulfiram for alcohol problems, or cholinesterase inhibitors for dementia—are discussed in the chapters that deal with the relevant clinical syndromes.
Anxiolytic drugs
Anxiolytic drugs, such as benzodiazepines, have been prescribed widely and often inappropriately. Before prescribing anxiolytic drugs it is always important to seek the causes of anxiety and to try to modify them. It is also essential to recognize that a degree of anxiety can motivate patients to take steps to reduce the problems that are causing it. Therefore removing all anxiety in the short term is not always beneficial to the patient in the long run. Anxiolytics such as benzodiazepines are most useful when given for a short time, either to tide the patient over a crisis or to help them to tackle a specific problem.
Tolerance is a particular problem with barbiturates and benzodiazepine-like anxiolytic drugs, and drug dependence can develop. Because the benzodiazepines are still widely used anxiolytics, they will be considered first. Anti-depressants are increasingly used to treat specific anxiety syndromes, but their therapeutic actions differ in important ways from benzodiazepine-like drugs. Their indications in the treatment of anxiety disorders will be considered here, but their detailed pharmacology is discussed in the section on antidepressant drugs (see p. 531). When reading this section, it is important to bear in mind that psychological treatments are effective in the management of anxiety disorders and have certain advantages over drug treatment, including more sustained efficacy after treatment cessation, as well as fewer adverse effects.
Table 19.1 Classification of clinical psychotropic drugs
Benzodiazepines
Pharmacology
Benzodiazepines have several actions:
• anxiolytic
• sedative and hypnotic
• muscle relaxant
• anticonvulsant.
Their pharmacological actions are mediated through specific receptor sites located in a supramolecular complex with gamma-aminobutyric acid (GABAA) receptors. Benzodiazepines enhance GABA neurotransmission, thereby indirectly altering the activity of other neurotransmitter systems, such as those involving noradrenaline and 5-HT.
Compounds available
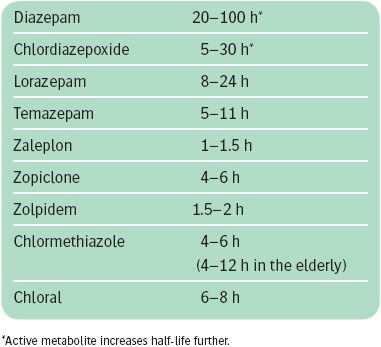
Many different benzodiazepines are available. They differ both in the potency with which they interact with benzodiazepine receptors and in their plasma half-life (see Box 19.3). In general, high-potency benzodiazepines and those with short half-lives are more likely to be associated with dependence and withdrawal. Benzodiazepines with short half-lives (less than 12 hours) include lorazepam, temazepam, and lormetazepam.
Because of problems with dependence, long-acting benzodiazepines are preferable for the management of anxiety, even if such treatment is to be given intermittently on an ‘as-required’ basis. Long-acting benzodiazepines include drugs such as diazepam, chlordiazepoxide, alprazolam, and clonazepam. Diazepam is rapidly absorbed and can be used both for the continuous treatment of anxiety and for treatment ‘as required.’ Alprazolam, a high-potency benzodiazepine, is effective in the treatment of panic disorder. This therapeutic efficacy is not confined to alprazolam, because equivalent doses of other high-potency agents such as clonazepam are also effective.
Flumazenil is a benzodiazepine-receptor antagonist that produces little pharmacological effect by itself, but blocks the actions of other benzodiazepines. Therefore it may be useful in reversing acute toxicity produced by benzodiazepines, but carries a risk of provoking acute benzodiazepine withdrawal. Flumazenil is available only for intravenous use.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree