Acraniocerebral trauma raises the important question of the degree of cerebral disturbance. This question may be answered easily in cases of commotio cerebri or slight concussion of the brain; this is a reversible impairment of consciousness of brief duration with no clinical evidence of any gross structural change in the brain substance. The question of brain function becomes more important in cases of cerebral contusion after more severe injury with prolonged unconsciousness and signs of brainstem dysfunction. Brainstem damage may result from primary brainstem injury (
1,
2) or from secondary brainstem lesion due to downward transtentorial herniation (
3). It has been shown that brainstem injury does not exist in isolation; it is merely one aspect of diffuse brain damage (
4).
The clinical parameter of the grade of disintegration of brain function and impairment of the brainstem is demonstrated by the development of an acute secondary midbrain and bulbar brain syndrome (BBS). The symptoms of the well-known rostrocaudal deterioration, first described by Mc-Nealy and Plum (
5) and Plum and Posner (
6) and modified by Gerstenbrand and Lücking (
7), allow a clear clinical statement about the depth of posttraumatic coma. In addition to the neurologic examination, cranial computed tomography (CT) and magnetic resonance imaging (MRI) easily identify a space-occupying lesion and sometimes demonstrates the displacement of the brainstem (
8,
9). These methods, however, fail to give any information about cerebral activity. Therefore, the electroencephalogram (EEG) is still important in the diagnosis of traumatic cerebral lesions, especially in the assessment of the degree of cortical activity, which shows reasonably good correlation with the depth of posttraumatic coma (
10,
11,
12,
13,
14,
15 and
16). Chronic stages of craniocerebral trauma demonstrate a diminished electroencephalographic-neurologic correlation (
17,
18,
19 and
20). However, there is an approximate correlation between the EEG and clinical improvement, especially in patients who have had systematic follow-up studies of EEG and clinical examination (
21,
22,
23 and
24). Occasionally, the EEG may return to normal when neurologic or psychiatric abnormalities persist, a disparity that indicates a bad prognosis (
18,
25,
26). By contrast, in some patients with normal clinical findings, an abnormal EEG may be the forerunner of an intracranial complication such as posttraumatic epilepsy (
21).
EARLIER EEG WORK IN CRANIOCEREBRAL TRAUMA
Williams (
27) studied a few patients after mild craniocerebral trauma and observed that normal records can be obtained within a few hours of injury. Further electroencephalographic studies in brain injuries were done by Dow et al. (
28), who included patients with more severe injuries and repeated studies during the recovery of the patients. Dawson et al. (
29) continued to observe patients and studied the evolution of abnormalities with repeated records. Extensive work in this field was done by Meyer-Mickeleit (
19), Schneider and Hubach (
30), Courjon and Scherzer (
21), and Koufen and Dichgans (
23).
The impairment of consciousness to any degree was usually found to be accompanied by an abnormal EEG (
19,
28,
29,
30,
31 and
32). Most of these authors directed their attention to the frequency of the basic activity and to focal, general, or epileptic abnormalities, but no further distinction of the EEG pattern was made. High-voltage delta activity was regarded as the electroencephalographic correlate of coma, which in turn was considered an equivalent of deep sleep (
33,
34 and
35). Further studies in coma (
36,
37 and
38) made it clear that states of coma were associated with a variety of EEG patterns, often with the criteria of sleep (
12,
14,
39). The manifestation of sleep patterns appeared to be of prognostic significance, supported by the results of overnight or prolonged EEG recordings (
40,
41 and
42). The role of reactivity in the assessment of the depth of coma was first pointed out by Fischgold et al. (
43) and is an important prognostic indicator for good outcome (
44). In addition to these findings, asymmetry in reactivity was considered a reliable lateralizing sign (
45).
Important reviews concerning posttraumatic comatose states have given detailed insight into the EEG in altered states of consciousness (
10,
46,
47). Early and late changes of the EEG after craniocerebral trauma were discussed extensively by Courjon and Scherzer (
21) and Stockard et al. (
48).
EXPERIMENTAL WORK
Williams and Denny-Brown (
49) found an immediate reduction in amplitude (sometimes approaching isoelectricity) in the EEG in cats subjected to experimental head concussion. After a short period of delta activity, the EEG returned to normal within a period of seconds to a few minutes. Dow et al. (
28) studied the physical factors responsible for the injury in humans. These factors appeared to be of the same order as those necessary to produce experimental concussion in animals (
50). Interestingly, the threshold velocity that provoked concussion at the moment of impact in animals also marked the limit between the average velocity in patients with borderline and abnormal EEG (
28). Other than this finding, pathologic observations made in humans could not be convincingly duplicated in experimental studies. This might be due to the smaller mass of brain in animals and the restriction in impact
techniques (
51,
52). Even in standardized animal experiments, the analysis of the EEG did not provide adequate indices of injury severity, and there was no correlation found with the duration of coma (
53).
Following the convulsive theory of slight concussion (
54), the energy imported to the brain may generate turbulent rotatory and other movements of the cerebral hemispheres provoking a convulsive disorder, which might present a mild form of cerebral concussion with a sudden short loss of consciousness, paralysis of reflex activity, and loss of memory (commotio cerebri). This theory may explain the observation that the EEG returns to normal within 24 hours in these cases.
Some closer correlations to EEG changes in comatose states in humans were found in other animal experiments producing localized destructive brain lesions or using local electrical stimulation. Local lesions in the white matter and in thalamic structures were immediately followed by the appearance of delta waves, which were accompanied by spindles in the case of thalamic lesions. Slow activity developed gradually in mesencephalic reticular formation lesions; it was absent in cortical lesions (
55). EEG changes similar to those seen in thalamic destructive lesions were found after stimulation of thalamic nuclei (
56,
57). Stimulation at the level of the mesencephalic reticulum induced behavior and EEG arousal in sleeping in otherwise intact animals, whereas destructive lesions at mesencephalic levels caused coma and EEG activity reminiscent of different types of sleep (
58). All these mechanisms may be involved in posttraumatic comatose states as long as delta waves and spindles are generated by otherwise relatively normal cortical neurons.
CLASSIFICATION OF EEG
Different parameters have been used in the breakdown of EEG signs in comatose states (
10,
11 and
12,
14,
21,
36,
60,
61 and
62). To increase the value of the EEG in posttraumatic coma, all these parameters were integrated and correlated with the stages of rostrocaudal deterioration (
13).
The EEG abnormalities may be classified into five grades (
13): grade 1, predominant alpha and little theta activity; grade 2, predominant theta and little delta activity; grade 3, predominant high-voltage rhythmic and arrhythmic delta and subdelta activity; grade 4, diffuse, mostly low-voltage delta and subdelta activity and low-voltage activity only recognizable with increased amplification (3.5 µV/mm); and grade 5, isoelectric record.
Other signs apart from these grades can also be noted in the EEG: (i) superimposed fast activity (6 to 18 c/sec localized over the frontal region or diffusely spread); (ii) normal-looking sleep records; (iii) diffuse slowing accompanied by “typical sleep potentials” (spindles, vertex sharp waves, K complexes); (iv) diffuse slowing accompanied by altered “sleep potentials,” listed under “atypical sleep potentials”; (v) spontaneously alternating EEG patterns with a rapid succession of low-voltage delta activity and high-voltage slow waves (this activity can be classified as delta bursts [0.5 to 2 seconds] and short [2 and 5 seconds] and long [more than 5 seconds in duration] runs of delta with frequencies of 0.5 to 4 c/sec); (vi) “lateralized signs” such as local slow activity (“local slowing”), unilateral predominant slow activity, unilateral low-voltage output, and unilateral depression of superimposed fast activity listed under the heading of “unilateral predominant slowing,” asymmetries of “typical or atypical sleep potentials,” and asymmetries of response to external stimuli; (vii) four types of reaction to external stimuli: (
1) appearance of widespread 1 to 7 c/sec activity, (
2) blocking of slow activity, (
3) appearance of alternating EEG patterns, and (
4) appearance or disappearance of “typical or atypical sleep potentials”; and (viii) epileptiform patterns: (
1) focal or generalized runs of spikes and sharp spike waves, (
2) periodic lateralized epileptiform discharges (PLEDs), and (
3) triphasic waves.
Reactivity may be immediate or delayed. Delayed reactivity is a slow EEG response inducing a new pattern under the influence of repetitive stimulation (
10). Fischgold and Mathis (
36) emphasize the usefulness of auditory and painful stimuli in contrast to the insignificant effect of visual stimulation. In lighter stages of coma, stimulation may also provoke extracerebral changes such as muscle activity and respiratory artifacts.
CLASSIFICATION OF COMA
Physiologically, the loss of consciousness implies that the patient has suffered widespread dysfunction of cerebral hemispheres or the brainstem or both. When there is a diffuse swelling of the brain or bilateral hemispheric lesions, the cerebral hemispheres, the diencephalon, and the adjoining midbrain are displaced downward through the tentorial notch. In this case, the herniation through the tentorium is symmetrical (central tentorial herniation). If downward displacement continues, the contents of the posterior fossa will finally be pressed through the foramen magnum. This brain shift evokes a number of characteristic clinical signs that have been described by various authors (
5,
6 and
7). The most prominent clinical signs of central herniation are listed in
Table 22.1. According to Gerstenbrand and Lücking (
7) posttraumatic comatose states can be broken down into six stages, including four stages of midbrain syndrome (MBS) and two stages of BBS. With regard to the terminology of Plum and Posner (
6), MBS 1 and MBS 2 correspond to the early diencephalic stage, MBS 3 to the late diencephalic stage, MBS 4 to the midbrain-upper pons stage, BBS 1 to the lower pontine-upper medullary stage, and BBS 2 to the medullary stage.
In cases with expanding lesions in the lateral middle fossa or temporal lobe, the medial edges of the uncus and hippocampal gyrus are commonly pushed toward the midline and over the free lateral edge of the tentorium, compressing the third cranial nerve (early uncal herniation). During uncal herniation, a unilateral dilated pupil is the most consistent finding (
6). Other neurologic signs may be present at this early stage and may provide hints concerning the original hemispheric lesion. Once the pupil dilates fully (late uncal herniation), the patients become comatose, and abnormal postures and motor responses evolve (
6,
63). Eventually, with increasing intracranial pressure, lateralizing signs disappear and the patients show the clinical signs of MBS 4 or BBS 1.
Neurologic signs different from this expected rostrocaudal deterioration may be found in cases of primary brainstem injury. Maciver et al. (
2) state that a primary brainstem lesion can be suspected if the neurologic signs do not fit the classical stages of rostrocaudal deterioration (i.e., the relatively intact oculomotor functions are in contrast to decerebrate posturing and respiratory abnormalities). The CT scan is diagnostic and eliminates a supratentorial lesion causing secondary brainstem dysfunction. The MRI scan shows the extent of brainstem damage in the further course.
RELATION OF EEG ABNORMALITIES TO THE STAGE OF COMA
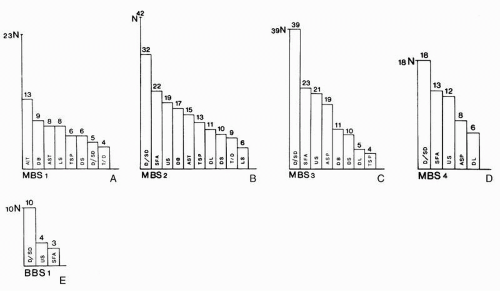
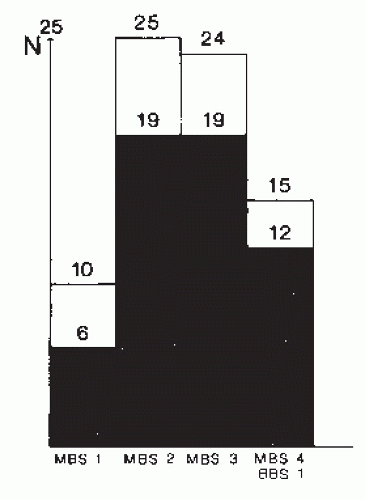
The EEG patterns in the different stages of MBS and BBS are presented in
Figure 22.1. The significance of this observation is somewhat hampered by the fact that the figure presents EEG data obtained within 1 week after brain injury, thereby neglecting the special behavior of spindle activity (see the section “
Relation of EEG Abnormalities to Outcome”). Nevertheless, the figure demonstrates well the principal relation of EEG to the stages of coma. There is a decrease in the number of different EEG patterns related to the stages of MBS and BBS, indicating the increase of intracranial pressure. Unfavorable prognosis is shown by the disappearance of “typical or atypical sleep
potentials,” alternating patterns, and loss of reactivity (
10,
12,
13 and
14,
21,
41). In accordance with the mildly diffuse cortical disturbance, the EEG is slightly or moderately abnormal in MBS 1.
Stimuli to diencephalic or rostromesencephalic structures (
64) may induce delta bursts or short runs of delta waves. Local slow activity can be seen only in an otherwise mildly abnormal EEG, while increasing diffuse slowing may overwhelm local abnormalities (
65). Borderline EEG patterns occur at MBS 1 but not in deeper stages of MBS. In MBS 2, the amount of slow activity increases. This increasing abnormality may be due to a direct cortical disturbance (
64) or due to a more remote effect from deeper structures (
64,
66). However, “sleep or sleep-like potentials” (see
Figs. 22.6A and
22.7A) indicate a relatively intact cortex (
14). In MBS 2, the EEG shows the widest range of different EEG patterns. In MBS 3, a clear reduction in the number of EEG patterns is noted. A decrease of spontaneous alternating patterns and “sleep potentials” has been thought to indicate an increasing disturbance of the diencephalic and mesencephalic systems (
12).
Increasing rostrocaudal deterioration is further characterized by the increased change of typical or atypical sleep potentials (
14) and the shift of superimposed fast activity, diffusely spread in lighter stages of MBS, to the frontal regions (
67). The EEG patterns are still more simplified in MBS 4 and BBS 1. The absence, reduction, and deterioration of sleep potentials or alternating patterns are suggestive of marked damage at the diencephalic level (
39,
41). The telencephalon (
10) must also be involved, considering the diffuse brain edema in advanced stages of MBS. In BBS 1, low-voltage delta and subdelta activity appears, followed by low-voltage cerebral activity, often recognizable only with increased amplification. No electrical cerebral activity can be observed in patients with BBS 2 (
13). Patients with an isoelectric EEG or an EEG with repeated isoelectric periods will die (
68). The high prognostic value of an EEG classification with a great number of different EEG patterns was supported by the development of an EEG score, which included many of these phenomena weighted according to their perceived prognostic value and scored as dichotomous variables— present or absent (
69).
Unreactive alpha activity or “alpha-like” rhythms (
70) are not seen in patients in BBS 1 after supratentorial lesions causing
secondary brainstem involvement. This pattern usually appears in polytraumatic patients at this stage and thereby strongly suggests an additional hypoxic or anoxic cerebral lesion. The same suggestion can be made with theta-pattern coma, which was found to be a variant of alpha coma (
71). Usually, both patterns are predictors of fatal outcome. Rhythmic coma in children especially in the alpha frequency range has generally a better prognosis than in adults (
72).
Primary brainstem injuries may produce clinical symptoms that are hardly separable from symptoms of secondary brainstem injury caused by downward herniation. Although primary brainstem damage rarely exists in pure form (
4), a primary brainstem lesion may be the principal cause of coma. In these cases, the EEG can be a helpful diagnostic tool. Patients in coma from caudally placed brainstem lesions may have nonreactive alpha activity even in an unresponsive decerebrate state (
73). Sleep spindles, vertex sharp waves, and K complexes suggest that the lesion is below the still intact thalamocortical system (
74). This suggestion has been confirmed by Steudel et al. (
75), who described brainstem lesions with evidence based on cranial CT and autopsy findings in all their decerebrate patients with spindles and/or alpha activity in the EEG. During the first week, these activities were replaced by delta waves, indicating that EEG patterns similar to the one generally seen in prolonged comatose states may appear (
46,
76).
The reaction to external stimuli further characterizes the depth of coma (
10,
21). In MBS 1, sensory stimulation may briefly block the slow activity; sleep, easily recognizable at this stage, may have a similar effect (
48). In further stages of MBS, reactivity consists of widespread delta activity, alternating patterns, and typical or atypical sleep potentials (see
Fig. 22.13A). In BBS 1 and BBS 2, no reactivity can be observed.
Generalized burst suppression occurs in posttraumatic coma only when cerebral anoxia was sustained with the trauma (
48,
67). Burst suppression activity due to an overdose of soporific drugs tends to produce uniform intersuppression activity, in contrast to the intersuppression activity in anoxic coma, in which delta waves and intermittent spikes are observed frequently (
48). This type of activity carries a poor prognosis because it indicates a diffuse anoxic encephalopathy that interferes with the original traumatic brain damage.
Metabolic derangements certainly have an important impact on coma and accompanying EEG abnormalities (
10,
13,
14,
39,
77,
78 and
79). The number of metabolic disturbances is obviously higher in patients in deep stages of posttraumatic coma (
13). Most of these patients suffer from multiple trauma including the liver, kidneys, and lungs. The combination of metabolic and traumatic encephalopathy carries the worst prognosis. The EEG changes due to metabolic disturbance interfere with the EEG changes because of the herniation itself, thus blurring the rules concerning the decrease of different EEG patterns with advancing deterioration. Further influences on the EEG are caused by the effects of sedative or anesthetic drugs frequently used in intensive care units.
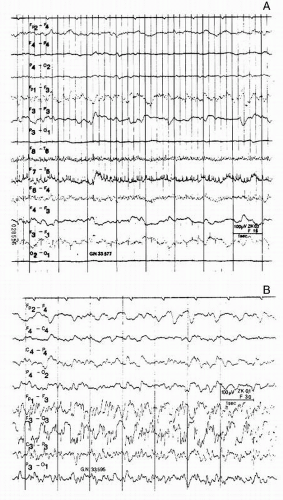
Early electroencephalographic seizure activity is only seen in cases of severe craniocerebral trauma, in which patients may have clinical seizures shortly before or shortly after the discharges are found in the EEG (
Fig. 22.2). Acute epileptic and paroxysmal EEG discharges may also accompany nonconvulsive (subclinical) seizures or status epilepticus in severe brain injuries. In cases of nonconvulsive seizures or status, a more severe clinical picture is simulated. Seizures either are generalized from the start or more
often are focal with subsequent generalization (
21). Focal seizure activity consists of runs of spikes and sharp waves and is more likely to occur in patients with underlying intracranial hematoma. The EEG is of considerable localizing value in these cases, and there has been only one reported case of focal seizure activity occurring contralateral to the posttraumatic hematoma (
21).
Usually, the appearance of the electroencephalographic seizure activity at these stages of coma carries a worse prognosis (
21) and is associated with a mortality that is about three times higher than in the absence of seizure activity (
48). Intracranial pressure may increase even in the presence of nonconvulsive subclinical status epilepticus or repetitive electroencephalographic seizures (
80). Status epilepticus associated with generalized motor seizures may be the result of severe cerebral trauma. Death occurs in more than 50% of these cases within the first few days (
21). In children, posttraumatic status epilepticus is associated with a far less ominous prognosis than in adults (
81). Isolated spikes and slow waves are rare after a recent injury (
82), but they may occur in severe cases. Although prognosis in cases with early seizures is generally bad, acute convulsions are a more serious complication in patients with brain tumor and metabolic encephalopathies (
83). Unfortunately, quantitative EEG has difficulties detecting spikes, brief seizures, burst suppression, PLEDs, triphasic waves, and intermittent theta and delta activity. Therefore, the use of serial standard EEG recordings at least three times a week is still required in intensive care patients (
84).
PLEDs are rare events in posttraumatic coma; they are usually seen in cases of subdural hematoma (
85). More frequent PLEDs are seen in patients with acute unilateral lesions of vascular origin (
86,
87). Triphasic waves may also be produced by subdural hematoma, but they are more characteristic of metabolic disturbances (
88,
89), especially when no asymmetry can be detected.
The lateralizing signs in the EEG (other than epileptic discharges) demonstrate a close correlation to the lateralizing signs of the neurologic examination (
Fig. 22.3). In MBS 4 and BBS 1, the neurologic examination reveals no lateralizing signs at all. At these stages, the EEG gives a hint of local cerebral lesion. The localized signs in the EEG may accompany all local cerebral lesions including the three major types of hematoma—subdural, epidural, and intracerebral (
Fig. 22.4). Asymmetry in reactivity (
45) is helpful to ascertain uncertain lateralizing signs in the acute stages of coma (see
Chapter 23;
Fig. 23.9); in prolonged comatose states, asymmetry often does not manifest itself unless the patient is stimulated (
48).