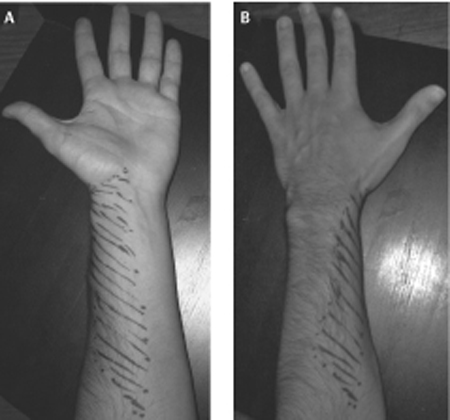
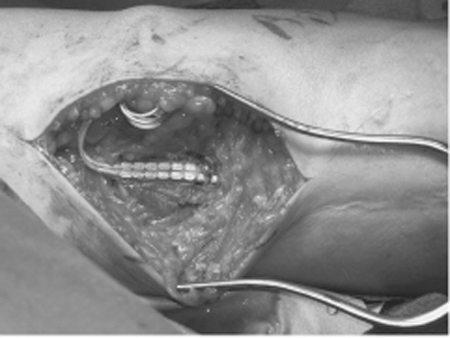
57 Electrical Stimulation for Painful Neuroma A 49-year-old man experienced shooting pain down the lateral aspect of his forearm during a venipuncture of the left cephalic vein. The pain initially resolved but recurred the next morning along with hyperalgesia over the lateral aspect of the forearm. On physical examination, palpation of the antecubital fossa provoked reports of pain shooting down the lateral aspect of the forearm to the base of the thumb. There was decreased pinprick sensation and brushing allodynia in the distribution of the lateral antebrachial cutaneous nerve (LACN). Electrodiagnostic studies confirmed complete injury of the LACN. Over the next 6 months the pain persisted and was refractory to pharmacological therapy including narcotics, tricyclics, and antiepileptic agents. At surgery, a neuroma in continuity of the LACN was observed. The nerve was first divided just proximal to the neuroma and then just distal to the neuroma, and the proximal end of the LACN was buried in the biceps muscle. After a 4-month period of complete relief, the patient returned to clinic with a recurrence of pain. A secondary surgical exploration revealed a neuroma of the proximal LACN. The neuroma was resected and a stimulating electrode was placed on the distal musculocutaneous nerve just proximal to the branch point of the LACN. After a trial period of 2 days, the optimal stimulation parameters were determined and the stimulator was permanently implanted. The patient had immediate pain relief, which has been maintained over a follow-up of 18 months to date. Lateral antebrachial cutaneous nerve neuroma treated successfully with an implanted electrical stimulator The LACN arises as a branch of the distal musculocutaneous nerve and contains sensory fibers primarily from C6 and C7. After the musculocutaneous nerve courses through the coracobrachialis muscle it gives off motor branches to the biceps and brachialis muscles. The remaining fibers (sensory) run deep to the biceps muscle and emerge at the anterolateral border of the bicipital aponeurosis. Here the nerve is vulnerable to compression by the sharp edge of the biceps tendon when the arm is flexed or pronated. The nerve enters the antecubital fossa on a course just below the cephalic vein, making it susceptible to needle stick during cephalic vein venipuncture. Other nerves that are also susceptible to needle stick include the cervical and brachial plexus in the region of the scalene muscles, the radial nerve in the arm, the axillary nerve in the quadrangular space, the sensory radial nerve at the wrist, and the sciatic nerve in the proximal buttock. At the elbow crease the LACN divides into anterior and posterior divisions and maintains a subcutaneous course providing sensory innervation to the radial forearm. Occasionally the LACN sensory distribution overlaps with the superficial radial nerve in the thenar area of the hand. A neuroma refers to a specific anatomical structure. When an axon is transected, the distal axon undergoes Wallerian degeneration, leaving behind an empty endoneural tube. In an effort to grow down the distal endoneural tube, the proximal axon forms sprouts that elongate. If the sprouts are thwarted by such factors as scar tissue, they form a local fibrotic tangle, the neuroma. A terminal neuroma can be the total end of a severed nerve or it can occur along the course of a nerve when only a portion of the axons were injured and are regenerating, enmeshed in scar (neuroma in continuity). Following peripheral nerve injury, afferent fibers whose regenerating axons become trapped in the nerve stump neuroma become hyperexcitable. This ectopic electrical activity is likely due to the accumulation, at the regenerating tip, of mechanical/chemical-to-electrical transducers and ion channels that are normally transported down the nerve fiber to the cutaneous endings. Nociceptive afferents that normally signal pain acquire spontaneous activity and heightened sensitivity to mechanical and chemical stimuli at the nerve stump. The constant barrage of impulses from the injured nociceptors leads to sensitization of central pain pathways and may underlie the ongoing component of neuromatous pain. The mechanical and chemical sensitivity at the nerve stump underlies the shooting, electrical pains elicited by changes in the local internal environment, or external pressure applied to the tissues overlying the neuroma (Tinel sign). LACN mononeuropathy is most commonly secondary to iatrogenic or traumatic injury. The nerve’s proximity to the cephalic vein makes it susceptible to needle stick injury during venipuncture, especially when the procedure is complicated and requires several passes to find the vein. The superficial course taken by the nerve in the forearm makes it vulnerable to laceration injury with glass or knives. Compression or trauma to the musculocutaneous nerve is a rare cause of LACN neuropathy. LACN mono-neuropathy has also been reported to occur in cases of neuralgic amyotrophy. When a nerve is punctured, the patient will often describe a sudden and severe electrical pain radiating in the distribution of the nerve alerting the health care professional to the injury. Over time the pain takes on a burning character. If the regenerating axons innervate a target, the pain often subsides. The diagnosis of neuropathic pain secondary to nerve injury can be made clinically in the majority of cases. Symptoms typically include shooting pain, electrical sensations, and burning pain in the distribution of the injured nerve. Neuropathic pain is almost always intensified by cooling stimuli and psychological stress. Patients will describe this as avoiding air conditioners or an open widow. They note that lack of sleep makes for an increase in the pain. In LACN injury, symptoms include shooting pain and sensory disturbances in the distal volar radial forearm. The area of sensory disturbances is limited to a 4 in. region in the distal third of the volar radial forearm (Fig. 57–1). Due to overlap of cutaneous nerves in the forearm, the sensory pattern may vary. Signs elicited on palpation of the lateral aspect of the cubital fossa include point tenderness and pain or paresthesias in the LACN distribution. Motor examination is expected to be normal. The Tinel sign, sensitivity to mechanical stimulation, occurs predominantly at the region of axonal sprouting. This location may proceed distally as the nerve regenerates (typically 1 mm/d) toward the target. Demonstrating advancement of a Tinel sign is often associated with a good prognosis. Figure 57–1 (A) Photographs of the anterior and (B), posterior views of the forearm. The lateral antebrachial cutaneous nerve innervates the area marked by dashed lines in the lateral and volar aspect of the radial forearm. There are many causes of extremity pain that are more common than neuropathic pain secondary to a neuroma. Tendonitis, fasciitis, epicondylitis, and arthritis of a joint can produce radiating pain. For example, tenosynovitis of the wrist extensor tendons (intersection syndrome) may produce radial wrist and forearm pain. Primary shoulder pathology can result in pain radiating down the arm toward the elbow. Neuropathic pain can also occur secondary to peripheral nerve entrapments or radiculopathy. Carpal tunnel syndrome typically produces symptoms in the hand but may also present as pain or paresthesias in the forearm and even an aching sensation throughout the upper extremity. Intervertebral disk herniation at C5–6 can produce radicular pain in the radial forearm and into the hand similar in distribution to carpal tunnel syndrome. The pain typically begins around the lateral aspect of the spine and radiates down the extremity. Finally, although psychogenic and secondary gain issues should be considered, especially in job-related injuries, these remain diagnoses of exclusion. The diagnosis of a painful neuroma is largely clinical. However, nerve conduction studies and somatosensory evoked potentials can be useful in the diagnosis of LACN injuries and other neuropathic pain conditions. It can be difficult to determine in a pure sensory nerve if an injury is neurapraxic or axonotmetic with electrical studies. For a motor nerve, due to the delay in Wallerian degeneration, studies are of marginal value in the first 2 weeks. The presence of active denervation in the muscle indicates an axonotmetic lesion. The advent of magnetic resonance neurography has been a significant improvement in the ability to image peripheral nerves. The technique is still being refined but will likely play an increasing role in the management of peripheral nerve pathology. Injury to the LACN results in a sensory loss; however, it is the presence of a painful neuropathic condition that will cause the patient to seek care. The goal of treatment is to eliminate or lessen the patient’s pain and suffering. Both pharmacological and surgical therapies are aimed at blocking ectopic firing from the afferent fibers trapped in the neuroma. The initial approach to managing patients with neuromatous pain is often pharmacological. In refractory cases, we recommend consultation with a pain clinic. Gabapentin (Neurontin, Pfizer, Inc., New York, NY) is the current drug of choice, and the starting dose is 300 mg t.i.d. and then titrate to effect up to a maximum dose of 1200 mg t.i.d. If after 6 to 8 weeks the patient reports no significant improvement, other regimens can be tried. These include other anticonvulsants such as phenytoin (Dilantin, Pfizer, Inc., New York, NY), carbamazepine (Tegretol, Novartis Pharmaceuticals Corp., East Hanover, NJ), lamotrigine (Lamictal, GlaxoSmithKline, Philadelphia, PA), tricyclic antidepressants (such as amitriptyline), or benzodiazepines. Opiates are added when the initial trials fail. There is much stigma over the use of opiates for nonmalignant pain, but one should not let this hinder the use of the drugs in select cases. Patients with complaints of cutaneous hyperalgesia/allodynia may benefit from topical applications of high-dose capsaicin (10%) or anesthetic agents such as lidocaine (Xylocaine, AstraZeneca Pharmaceuticals LP, Wilmington, DE; Lidoderm, Endo Pharmaceuticals, Chadds Ford, PA). Patient reassurance and education are an important component of rehabilitation. Additional benefit may be obtained by addressing psychosocial factors contributing to the painful condition. We recommend consultation with a pain psychologist and therapies such as cognitive behavioral therapy, behavioral modification, and biofeedback. Other nonoperative measures, including acupuncture, physical therapy, hypnosis, nerve blocks, and transcutaneous electrical nerve stimulation (TENS), may benefit selected patients. Surgery can be an option for patients with neuromatous pain that is refractory to non-operative management. The initial surgical approach is neurectomy to remove the painful neuroma. Once the neuroma is resected, the stump is repositioned from its superficial location and, if possible, buried in muscle or bone. Invariably, a new neuroma will form at the distal end of the nerve, making possible a resurgence of the pain. Burying the nerve stump deep in soft tissue or bone shields it from repetitive mechanical trauma and may alter neuroma formation by limiting scar tissue formation or partially inhibiting regeneration. Unfortunately, despite operative efforts, a percentage of patients will continue to have persistent pain and may require a second or even multiple operations. Electrical stimulation of peripheral nerves with implanted devices for pain control has been performed and developed over the past 4 decades. The most common peripheral nerves treated with peripheral nerve stimulation (PNS) include the median, radial, ulnar, posterior tibial, and common peroneal nerves. PNS has proved to be most successful in treating chronic pain due to nerve entrapment or trauma with less impressive results in treating sciatica, low back pain, or metastatic disease. Additionally PNS is frequently used in sympathetically maintained pain, complex regional pain syndrome (CRPS), and extremity burns. Peripheral nerve stimulation produces nonpainful paresthesias in the distribution of the nerve being stimulated, which are often described by patients as a “buzzing” sensation. The exact mechanism by which this provides pain relief has not been elucidated. In 1965 Melzack and Wall proposed the hypothesis that the perception of pain in the periphery is dependent on the balance of inputs reaching dorsal horn neurons from large-diameter myelinated afferents signaling touch and small-diameter afferents signaling pain. In painful states the input from small nociceptive afferents is greater than that from large-diameter afferents, and pain is perceived. They proposed that electrical stimulation of large fibers would decrease pain perception by either directly inhibiting activation of small nociceptive neurons or by significantly increasing large fiber input to the dorsal horn, thus “closing the gate” to chronic pain. The first clinical application of PNS was reported by Wall and Sweet. As a direct test of the gate control theory, they placed temporary electrodes on the infraorbital nerve in a patient with chronic facial pain and reported symptomatic relief. Several lines of evidence question the validity of the gate control theory: (1) coactivation of large fibers has little impact on normal pain sensibility; (2) low-frequency stimulation (5 Hz) of large-diameter afferents evokes pain in neuropathic pain conditions; (3) large-fiber neuropathies as a rule are not associated with pain. Hence much research is needed to further clarify the mechanism of pain relief. Accurate patient selection is essential for successful pain relief with peripheral nerve stimulation. In our practice, peripheral nerve stimulation is reserved for cases of chronic neuropathic pain due to peripheral nerve entrapment, or trauma that is not alleviated by nonoperative care or surgical resection of the neuroma. Criteria for selection of patients should include (1) pain localized to one nerve, (2) complete relief of symptoms after peripheral nerve block with lidocaine (Xylocaine) or bupivacaine (Marcaine, AstraZeneca Pharmaceuticals LP, Wilmington, DE), (3) satisfactory results on psychological or psychiatric assessment, and (4) no exacerbation of symptoms with TENS. The indications for PNS over spinal cord stimulation (SCS) are unclear. In our practice, we often reserve PNS for patients who had a good response to SCS but then failed secondary to electrode/coverage issues. It is much easier to trial an SCS than a PNS system and the SCS does not have issues with motor stimulation that the PNS has. It is only in the upper extremity and a pure sensory nerve that we would go directly to PNS. A two-stage procedure is performed for placement of a peripheral nerve stimulator. The first stage consists of a trial with a temporary electrode to determine if PNS would provide pain relief. Under general anesthesia, the nerve to be stimulated is dissected free of adjacent structures and scar tissue. If indicated an internal neurolysis or neuroma resection and relocation (as described earlier) is performed. For a nerve containing both sensory and motor fibers, direct nerve stimulation is performed to differentiate between motor and sensory fascicles. The stimulating electrode should be placed closest to sensory fascicles to maximize stimulation of sensory fibers and minimize adverse stimulation of motor fibers. This is best accomplished more distally in the nerve where there is better separation. A small, thin piece of local fascia or muscle can be placed on the nerve proximal to the apparent site of nerve damage to prevent direct contact between the electrode and nerve. An electrode is then placed atop the soft tissue barrier. In our practice the electrode is placed directly on the nerve (Fig. 57–2). The electrode is sutured to adjacent fascia or muscle to prevent migration. A temporary extension cable is attached to the electrode, passed through a subcutaneous tunnel to exit percutaneously, and secured with a nylon suture. Electrical stimulation parameters are tested over the next 2 to 7 days. A computer-based program is used by the patient that automatically changes amplitude, rate, and pulse width of stimulation and records the patient’s location and intensity of stimulation and pain relief. A beneficial result can be anticipated when the patient experiences a light, not painful tingling in the peripheral nerve distribution. Once a setting is identified that provides optimal coverage and relief, the patient proceeds to the second stage of surgery.
 Case Presentation
Case Presentation
 Diagnosis
Diagnosis
 Anatomy
Anatomy
 Characteristic Clinical Presentation
Characteristic Clinical Presentation
 Differential Diagnosis
Differential Diagnosis
 Diagnostic Tests
Diagnostic Tests
 Management Options
Management Options
 Surgical Treatment
Surgical Treatment
Peripheral Nerve Stimulation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


