Fig. 1
World map shows distribution of Japanese encephalitis. http://gamapserver.who.int/mapLibrary/Files/Maps/Global_JE_ITHRiskMap.png
JEV affects children in the endemic areas because of herd immunity in the adults, whereas both adults and children are affected in newly invaded areas because of lack of immunity. The travelers to endemic areas may have severe illness because of absence of protective antibodies. The age specific attack rate is the highest between 3 and 6 years, which has been attributed to exposure of poorly clothed village children to mosquito bites. Most of the JEV infection in humans is subclinical. The estimated ratio of symptomatic to asymptomatic infection ranges between 1:25 and 1:1,000 (Solomon and Winter 2004).
2.2 Virus
JEV is a single stranded positive sense RNA virus wrapped in a nucleo-capsid which is surrounded by a 50 nm glycoprotein containing envelope. The viral RNA has 5′ untranslated region (UTR), a longer 3′ UTR and an open reading frame in between (Chambers et al. 1990). JEV genome RNA encodes three structural proteins—(1) Capsid protein (C), (2) Precursor to membrane protein (prM), (3) Envelope protein (E); and seven nonstructural proteins (NS) NS1, NS2a, NS2b, NS3, NS4a, NS4b and NS5. The E glycoprotein is the most immunogenic protein, composed of 500 amino acids, and the main component of the surface projection of the virion. E protein is responsible for induction of neutralizing antibodies and accounts for the protective response in the host. E protein is also regarded as the cell receptor binding protein and mediates cell fusion and cell entry. The flavivirus enters the cell by endocytosis after attaching to a heparan sulfate receptor. Subsequently the lipid membrane of virus fuses with endosome membrane. The viral RNA enters the cytoplasm of the host cell (Chambers et al. 1990). The virulence of JEV depends on E protein and change in any amino acid may alter its virulence (Cecilia and Gould 1991; Ni and Barrett 1996).
2.3 Life Cycle of JEV
JE is a zoonotic disease and is transmitted between mosquitoes, pigs and water birds (Fig. 2). Humans are accidentally infected by the bite of JEV infected mosquitoes and are dead end host. Humans do not participate in the spread of JEV because of short and low level viremia. Pigs and ardeid birds are the most important host for the maintenance, amplification and spread of JEV. The pigs are the main participants in the transmission cycle with respect to humans. JEV infected animals usually remain asymptomatic though fatal encephalitis has been reported in horses and fetal losses in sows (Burke and Leake 1988). The bird mosquito cycle is thought to be important for maintaining and amplifying JEV in the environment. Culex mosquitoes, especially Culex tritaeniorhynchus, is the most important vector for JEV transmission. C. tritaeniorhynchus breeds in the rice field with shallow water, can fly up to 1.5 km horizontally and up to 1.5 m vertically (Asahina and Noguchi 1968; Scherer et al. 1959).
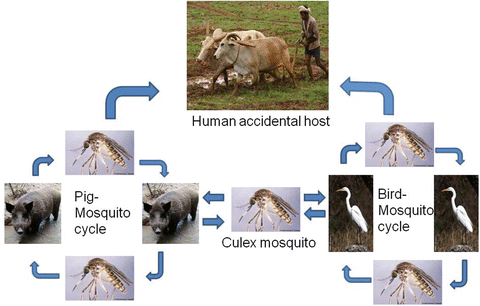

Fig. 2
Life cycle of Japanese encephalitis virus (with permission from http://www.encephalitisindia.org)
2.4 Pathogenesis and Pathology
Following an infected mosquito bite, JEV multiplies in the dermis and then in lymph nodes. Subsequently there is a short viremia during which the virus enters the brain. JEV multiplies in the endothelium of cerebral vessels and thus crosses the blood-brain barrier (Dropulie and Masters 1990). JEV replicates in the neurons. There is perivascular cuffing and infiltration of inflammatory cells, and phagocytosis of neurons (Johnson et al. 1985; Miyake 1964). In fulminant cases, evidence of inflammation may be lacking but viral antigen may be demonstrated in morphologically normal neurons. This may account for normal CSF in some patients. At autopsy, the lesions are mostly restricted to thalamus, basal ganglia, midbrain, cerebral cortex, cerebellum and anterior horn cells of spinal cord. In the acute stage, histopathological examination reveals areas of congestion, small hemorrhages, thrombi formations and neuronophagia. In the sub-acute stage, degeneration, loss of neurons and proliferation of glial cells are more prominent whereas inflammatory cell infiltration is not obvious. In the chronic stage, degeneration or loss of neurons, gliosis, and inflammatory cell infiltration are apparent. In the late stage, there may be calcification of basal ganglia, thalamus and cerebral cortex. The highest concentration of JEV RNA and antigen has been demonstrated in thalamus and brainstem (Srivastava et al. 2012a, b). In the cerebellum, JEV antigen is localized to neurons in the granular and molecular layers, but not to Purkinje cells (Johnson et al. 1985). Co-infection of cysticercosis with JE has been reported in up to 30 % of the patients (Shankar et al. 1983).
2.5 Immunology
Following primary JEV infection, a rapid IgM response occurs in serum within 7 days, and by 1 month, IgM level declines and IgG level increases. Both symptomatic and asymptomatic JEV infections have IgM and IgG response in serum, but only JE patients have this response in the CSF. In the patients who had an earlier flavivirus infection, there is an early rise of IgG and slow rise of IgM. These patients may have milder illness due to neutralization of extracellular viruses and lysis of infected cell through antibody dependent cytotoxicity (Carmenaga et al. 1974). Persistence of JEV in the nervous system has been reported in 5 % of laboratory confirmed cases of JE (Ravi et al. 1993).
The cellular immune response probably restricts the viral replication before it invades the central nervous system (CNS). Immunosuppression by cyclophosphamide results in rapidly progressive encephalitis following JEV infection (Nathanson and Cole 1970). It is therefore likely that cytotoxic lymphocytes help in clearance of JEV. Neuronal injury and host defense in JE have been attributed to a number of pro-inflammatory cytokines and chemokines, which are released by activated microglia and astrocytes in the brain. Cytokines such as tumor necrosis factor (TNF)-α and interferon (IFN)-α/β, -γ activate intracellular antiviral pathways after binding to specific receptors on the surface of infected and uninfected cells. Other cytokines also contribute to antiviral responses (Burke and Morrilnl 1987; Ghoshal et al. 2007). In clinical studies, however, pro-inflammatory cytokines and chemokines were not related to disease severity and mortality (Kalita et al. 2010; (Solomon and Winter 2004). In JE patients with movement disorders, CSF norepinephrine, dopamine, 3,5 dihydroxyphenyl acetic acid, serotonin and homovalinic acid were reduced (Kalita et al. 2007). In an experimental study these neurotransmitters and metabolites were decreased in thalamus, striatum, midbrain and cortex (Misra et al. 2010a). In experimental studies, the maximum damage of thalamus and midbrain in JEV infection was demonstrated by documenting maximum number of JEV RNA copies, JEV antigen labeling and cytokine expression in these areas (Kumar et al. 2009a; Misra et al. 2010a; Srivastava et al. 2009, 2012b).
2.6 Clinical Features
The incubation period of JEV in humans is 5–15 days. The clinical picture of JEV infection ranges from febrile headache to aseptic meningitis to severe encephalitis. Following 2–4 days of nonspecific fever, headache, gastrointestinal disturbance, the encephalitic illness follows. The encephalitis manifests with fever, behavioral abnormality or altered sensorium. Seizures, focal neurological deficit, decerebration, and tremulous eye movements may be associated. Cranial nerve palsy is rare. Severe cases with JE may develop respiratory paralysis which needs artificial ventilation.
2.6.1 Seizures
Seizures may occur in 6.7–47.2 % of the patients with JE, especially children (Gourie Devi et al. 1995; Kalita et al. 2003; Misra and Kalita 2001; Misra et al. 2008; Solomon et al. 2002). Seizures are focal with secondary generalization and may rarely progress to status epilepticus. High frequency of seizures resulting in poor outcome has been reported from Vietnam (Solomon et al. 2002).
2.6.2 Acute Flaccid Paralysis
JE is an encephalomyelitis and the patient may have varying degree of anterior horn cell involvement ranging from patchy focal reflex loss, muscle weakness to wasting simulating polio-like illness (Misra and Kalita 1997; Solomon et al. 1998). Anterior horn cell involvement in JE patients has been documented by electromyography, nerve conduction, motor evoked potential (Misra and Kalita 1997) and autopsy studies (Zimmerman 1946). Polio-like illness due to JEV infection without encephalitis has been reported from Vietnam but in our experience all the patients with anterior horn cell involvement have associated encephalitis.
2.6.3 Movement Disorders
Movement disorders are common in JE and are noted as the patient recovers from coma (Dickerson et al. 1952; Misra and Kalita 1997, 2010). In a study, 74 out of 209 encephalitis patients developed movement disorders. Among the patients with movement disorders 62.2 % had JE, 8.1 % had dengue, 1.4 % had miscellaneous and 28.3 % had nonspecific encephalitis (Misra and Kalita 2010). Two major types of movement disorders are noted in JE:
1.
Parkinsonian features—characterized by mask-like face, abulia, akinesia, rigidity with or without tremor.
2.
Dystonia: Nearly all types of dystonia occur in JE and include oromandibular, limb and axial dystonia (Kalita et al. 2011). The dystonia may be fixed or kinetic, mild to severe and sometime simulate status dystonicus (Kalita and Misra 2002a). The movement disorders in JE are usually refractory to medical treatment but regress spontaneously with time.
2.6.4 Laboratory Findings
There may be peripheral leucocytosis and hyponatremia. CSF reveals lymphocytic pleocytosis (10–100/mm3), protein increase (50–200 mg/dl) and normal glucose. In early stage, CSF may reveal polymorphonuclear pleocytosis.
2.6.5 Imaging
Cranial CT scan is abnormal in about 55 % of the hospitalized patients with JE and reveals thalamic and basal ganglia hypodensity with evidence of hemorrhage occasionally. MRI, however, is more sensitive than CT scan and reveals changes in up to 94 % of the hospitalized patients with JE. The MRI findings are present in thalamus in 94 % (Fig. 3), basal ganglia in 35 %, midbrain in 58 %, pons in 26 % and cerebellum or cerebral cortex in 19 % of the patients (Kalita and Misra 2000; Misra et al. 1994). In addition to thalamus and midbrain lesions, temporal lobe involvement in JE has been reported in 17.7 % of the patients (Handique et al. 2006). Newer MRI sequences have revealed greater sensitivity of FLAIR sequence in demonstrating the pathological changes in JE (Fig. 2) (Misra et al. 2010b). In the acute stage, single photon emission computed tomography reveals hyperperfusion (Kimura et al. 1997) whereas in the sub-acute and chronic stage there is hypoperfusion of thalamus, basal ganglia and frontal area (Kalita et al. 1999; Misra et al. 2010b).
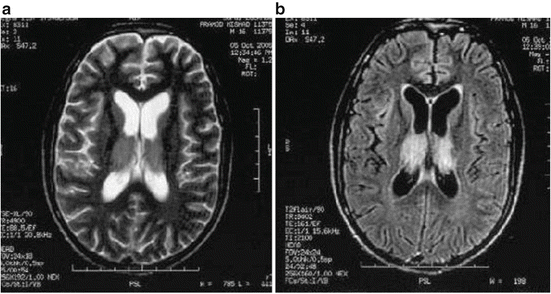

Fig. 3
Cranial MRI in axial section shows bilateral thalamic lesion on T2 sequence (a) which is more apparent on FLAIR sequence (b)
2.6.6 Electrophysiology
Electroencephalography (EEG) reveals non-specific slowing of delta to theta range. There may be lateralized epileptiform discharges and rarely alpha coma. The EEG changes improve within 1–3 months as the consciousness improves (Kalita and Misra 1998).
Nerve conduction studies are normal, except for compound muscle action potentials, which may be reduced due to loss of anterior horn cells. Electromyography reveals fibrillations and sharp waves with reduced interference. Anterior horn cell involvement on the basis of such findings was reported in 35.4 % of the patients (Misra and Kalita 1997). Pyramidal tract involvement results in abnormal central motor conduction in 73.9 % of the patients. Interestingly, the somatosensory evoked potentials are normal in spite of the high frequency of thalamic involvement in JE (Kalita and Misra 2002b).
2.6.7 Diagnosis
Thalamic lesions on CT or MRI are highly suggestive of JE in a patient with acute encephalitis in an JE endemic area. The confirmation of JE is based on detection of antibody, antigen, viral RNA, virus isolation and culture. Currently CSF IgM capture enzyme linked immunosorbent assay (ELISA) is the most acceptable method for the diagnosis of JE with 90 % specificity (Burke et al. 1985). The sensitivity of IgM capture ELISA is 75 % in the first few days of illness and increases to 95 % by the end of first week (Burke et al. 1985). In the early stage of disease, CSF JEV antigen (Ravi et al. 1989) and JEV RTPCR may be detected. Virus isolation from blood is difficult because of low level and short viremia. At autopsy, JEV can be isolated from the CSF and brain The yield of different laboratory tests of JE has been reported as 22.7–32.6 % for hemagglutination inhibition, 40–88.2 % for immunofluorescence, 47–68.2 % for MAC ELISA, 98.3 % for MACDOT, 86.5 % for PCR and 78 % for microneutralization (Tiroumourougane et al. 2002). This variability may be due to sampling time, type of specimen and clinical course of JE.
2.6.8 Differential Diagnosis
In the acute stage, JE may be difficult to differentiate clinically from other encephalitides. The absence of systemic manifestations helps to narrow down the possibilities. Herpes simplex virus (HSV) encephalitis has to be differentiated from JE as it has specific antiviral therapy. HSV encephalitis can be differentiated by characteristic fronto-temporal involvement on CT/MRI and positive PCR for HSV. Acute disseminated encephalitis may be differentiated by multiple white matter lesions. For details on differential diagnosis, see http://www.encephalitisindia.org.
2.6.9 Management
There is no specific antiviral treatment for JE. IFN-α and ribavirin have been found ineffective in clinical trials (Kumar et al. 2009b; Solomon et al. 2003). Corticosteroids do not reduce mortality or affect the outcome (Hoke et al. 1992). General care and symptomatic treatment are the same as in other encephalitides.
2.6.10 Outcome
About 20–40 % of the patients with JE die in the acute stage and 40–50 % of the survivors have sequelae, which include cognitive impairment, behavioral abnormality, focal weakness, movement disorders and seizures. Poor prognostic predictors are great age, high fever, deep coma, hypotonia, seizures, raised intracranial pressure and low level of IgG and IgM (Kumar et al. 1990; Misra et al. 1998; Solomon et al. 2002). The MRI changes, however, are not correlated with the outcome (Kalita and Misra 2000).
2.7 Prevention
There are two main strategies for prevention of JE
Immunization
Interfering with enzootic cycle
Immunization is the main way of prevention of JE. There are three types of JE vaccines (1) Formalin inactivated (2) Live attenuated and (3) Chimeric. Live attenuated vaccines are safe and effective and efficacy and safety of chimeric vaccine are under evaluation (Table 1).
Table 1
Vaccines against JEV infection
Type | Dose | Seroconversion | Side effects |
|---|---|---|---|
Formalin inactivated | 0.5–1 ml SC on 0, 7 and 30 days then yearly till 10 years | 91 % | 2–20 % (urticaria, angioedema, myalgia, headache, encephalomyelitis, seizure, neuropathy) |
Life attenuated | |||
SA14-14-2 | 0.5 ml SC booster every 5 years | 80 % after single dose, 98 % after two doses | Safe |
Vero cell | 0.5 ml I/M, 0 and 28 days | High | Safe |
Chimeric vaccine | Single dose | Under evaluation | |
Human exposure to mosquito bites can be reduced by wearing proper clothes, remaining indoor during dusk and dawn, using mosquito nets and distancing human habitat from rice fields and pigsties.
3 Dengue
3.1 Epidemiology
Dengue is regarded as the second most important mosquito-borne disease after malaria. The incidence of dengue has multiplied many folds in last 50 years. About four billion people live in dengue endemic areas of Asia, Africa, Australia, the Americas and Southern Europe (Fig. 4). Recently about 100 million patients with dengue fever (DF), 500,000 patients with dengue hemorrhagic fever (DHE)/dengue shock syndrome (DSS) and more than 25,000 deaths have been reported yearly (Brady et al. 2012; WHO 2009) (Fig. 5).
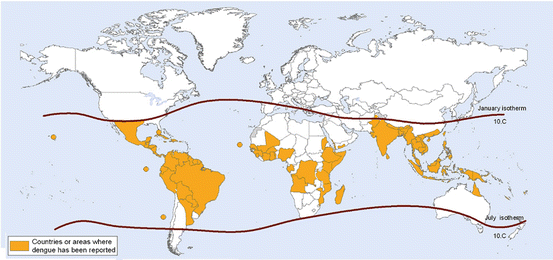
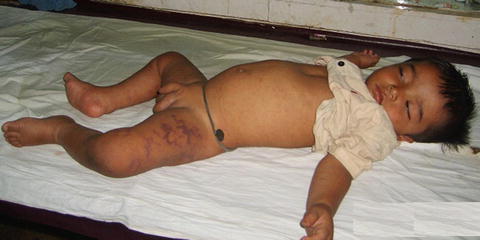

Fig. 4
World distribution of dengue virus infection (http://gamapserver.who.int/mapLibrary/Files/Maps/Global_DengueTransmission_ITHRiskMap.png)

Fig. 5
Photograph of a child with dengue hemorrhagic fever showing ecchymosis
3.2 Virus
Dengue virus (DENV) is a positive-strand RNA virus, about 15 nm in diameter and 100 Kb in length. The RNA genome is composed of seven non-structural protein genes (NS) and three structural protein genes encoding the capsid, membrane and envelope protein, respectively. The dengue virus serological group belongs to the family Flaviviridae and has four serotypes: DEN1, DEN2, DEM 3 and DEN 4. Although there is cross reactivity in these serological groups, infection by one serotype does not provide immunity to other serotypes; hence an individual living in an endemic area has the possibility of four attacks of dengue virus infection.
The DENV may have originated in Africa about 1,000 years back and might have migrated to South East Asia as all four serotypes of dengue virus are isolated from this region. DEN I serotype was isolated during the Second World War in the Pacific region by American and Japanese investigators. DEN-2 was isolated later. DEN 3 and DEN 4 were isolated in 1950s during the outbreaks in The Philippines and Thailand. The dengue serotypes have been classified into different genotypes based on sequencing E gene or from the junction of E and N genes. DEN 4 has three genotypes and DEN 1 and 2 have five genotypes. The epidemic potential and virulence of DENV depends on the genotype (Lewis et al. 1993; Rico-Hesse 1990). The transmission of dengue from Asia to other regions of the world might have occurred due to migration of individuals and increase in travels to new areas.
3.3 Life Cycle of DENV
Initially DENV was maintained in canopy dwelling Aedes mosquito and lower primates. Following deforestation, the mosquitoes have migrated to urban areas as they breed in shallow water, air cooler, flower vas, tiers etc. DENV has adapted to mosquito-human-mosquito cycle without needing any other animal for transmission. A. aegypti is an efficient vector for DENV transmission as it is widely available in tropics and subtropics, breeds close to human habitats, is highly anthrophilic and can transmit to more than one person in a single gonotrophic cycle. The other mosquitoes involved in the transmission of DENV are A. albopictus and A. polynesiensis. These mosquitoes are capable of transmitting DENV to their progeny. After feeding on a dengue infected person, a mosquito can transmit the infection to another person after an incubation period of 10–14 days. The infected mosquito remains infective for life. Humans are the main amplifying host of dengue although monkey also serves as reservoir in some part of the world.
3.4 Virus Entry and Replication
The monocyte, macrophage and dendritic cells are the primary cellular targets of DENV (Balsitis et al. 2009). DENV is attached to the cells by binding to DC-SIGN, CD209, C type lactin and mannose receptor. It enters the cells through clathrin-mediated endocytosis. Inside the cell, the viral nucleocapsid is released into the cytoplasm primarily by the help of anionic lipid which is released from the endosomes (Laughlin et al. 2012). This is followed by protein translation, viral RNA replication, immature virus assembly and viral maturation. Numerous mature viruses are released into the circulation to infect more target cells.
3.5 Pathogenesis
Following DENV infection, three major changes determine the clinical findings (a) increased vascular permeability, (b) thrombocytopenia, and (c) coagulopathy. With abatement of fever, there is increased vascular permeability due to high concentration of soluble IL2, IFNγ, IL2 receptor, CD4, CD8, CCL2 and vascular endothelial growth factor. The RNA load and NSI antigen determine the disease severity (Wang et al. 2006). The increase in vascular permeability in dengue results in hypotension. Shock in dengue is due to venous pooling. If the hypotension is not corrected, the diastolic blood pressure rises and pulse pressure narrows. Finally both systolic and diastolic blood pressures fall abruptly.
Increase in activated partial thromboplastin time and reduction in fibrinogen level along with thrombocytopenia correlate with the severity of dengue infection. Reduced fibrinogen is due to direct interaction of virus with plasminogen. Release of heparin sulfate and chondroitin sulfate from glycocalyx may also contribute to coagulopathy. In DENV infection, coagulopathy is usually minor and resolves in a few days as the virus is cleared. In the patients with DSS, the coagulopathy may be compounded by prolonged hypotension and tissue hypoxia leading to hemorrhage possibly due to disseminated intravascular coagulation. DHF and DSS are more commonly seen in secondary DENV infection and are attributed to two mechanisms:
(a)
Antibody-dependent enhancement (ADE): The neutralizing antibody-developed after the primary DENV infection declines over time. During the secondary DENV infection, binding of cross reactive neutralizing antibody at subneutralizing concentration enhances the infection of monocytes and dendritic cells via Fc receptors which is known as ADE and results in severe dengue (Laughlin et al. 2012).
These postulations however do not explain the occurrence of DHF/DSS in primary DENV infection suggesting the role other host and viral factors.
3.6 Clinical Features
The incubation period of DENV infection is 2–7 days. Most of the DENV infections are asymptomatic and the symptomatic patients clinically present with undifferentiated fever, dengue fever (DF), dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). The patient with dengue fever may manifest with muscle pain, anorexia, gastrointestinal symptoms, conjunctival congestion, lymphadenopathy and skin rash. Dengue hemorrhagic fever is associated with thrombocytopenia, evidence of plasma leakage in addition to fever and hemorrhagic manifestations. Association of DHF with shock is classified as DSS. In most of the patients with DSS consciousness is retained. The duration of shock is usually short, either the patient recovers following fluid replacement or dies within 12–24 h. Once the shock improves, the patient recovers in 2–3 days. The WHO guideline for case definition of dengue is presented in Table 2 (WHO 2009).
Table 2




Clinical case classification of dengue (World Health Organization 2009)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








