Fig. 17.1
(a, b) Classification of sphenoid wing meningioma (SWM). (a) I – Medial SWM without cavernous sinus (CS) involvement; II – medial SWM with CS involvement; III – middle SWM; IV – lateral SWM. (b) V – Osseous SWM without CS involvement; VI – osseous SWM with CS involvement; VII – true intraosseous SWM
17.3 Clinical Presentation
Figure 17.2 shows the predominant symptoms at the time of initial evaluation depended on classification of SWM. The symptoms were most commonly visual disturbance (highest in groups 1 and 2 with 66.7 and 60.9 %, respectively) and headaches, regardless of their predominant location. Double vision was complained in 30.4 % of patients in group 2 compared to only 5.1 % in group 1. The average length of history of these patients until the first consultation with a physician ranged from 1 to 360 months with a mean of 26.45 months, but decreased over the years with frequent and early use of sophisticated diagnostic investigations (1992–2002, 24.07 months, range 1–96; 1980–1991, 38.03, range 1–360 months).
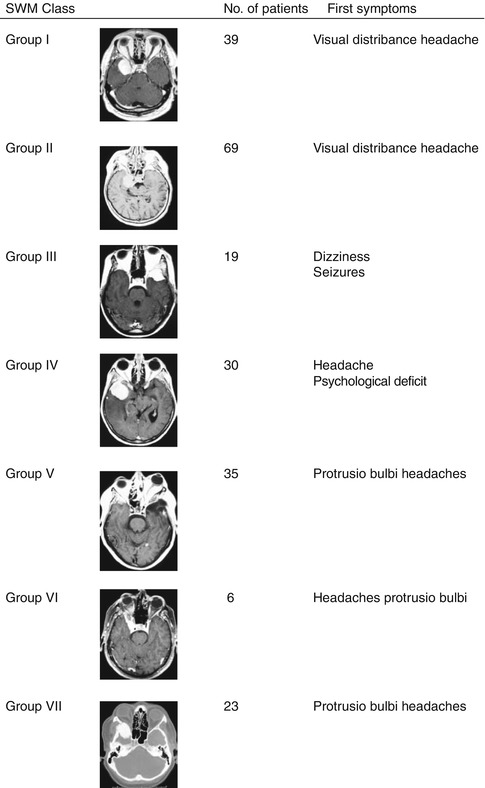

Fig. 17.2
Classification of SWM with their predominant clinical symptoms at the time of diagnosis
17.4 Radiological Presentation
There was bony involvement in 41 % of cases in group 1 tumours and 42 % in group 2 tumours. Rate of hyperostosis or intraosseous involvement was not different between all groups. Increase of the internal carotid artery and its branches was observed in the majority of cases within the medial SWM (group 1, 2 and 6). Peritumoural oedemas on CT or MRI scans were observed in 22 % of SWM with intraosseous involvement, in 31 % within group 1 and 52 % within group 2 tumours. Tumour calcification was significantly higher in osseous SWM (41 %) than in patients with group 1 and 2 tumours (25.6 and 21.7 %, respectively). Additionally gadolinium-enhanced MRI images showed enhancement within the bone in the majority of cases, reflecting true intraosseous tumour masses.
17.5 Surgical Considerations
Surgery is performed with general anaesthesia using an operating microscope and microsurgical instrumentation in all cases. For microsurgical tumour removal, the frontolateral and the pterional approaches are performed, techniques well described elsewhere (Fig. 17.3) [3, 14, 15]. For the frontolateral approach, a single burr hole is placed just posterior to the anterior temporal line using a high-speed drill, preventing to open the periorbita. The frontolateral approach provides a more medial view of the clinoid and suprasellar regions and thus early identification of the optic nerve in clinoidal meningiomas. For the pterional approach, the single burr hole is placed at the same location, but the craniotomy is performed more posteriorly, exposing the frontal and temporal lobe with the sylvian fissure and the sphenoid ridge. The lesser wing of the sphenoid is resected to the superior orbital fissure, depending on the tumour size, to allow exposure of the lesion with minimum brain retraction. The superior orbital fissure is similarly unroofed to expose the periorbital fascia, which is only opened in cases of obvious tumour infiltration of intraorbital structures. If there is marked hyperostosis around the optic canal, it is mandatory to perform first a partial clinoidectomy, then unroofing of the optic canal extradurally. The dura of the region of the tuberculum sellae and chiasmatic sulcus is carefully inspected for evidence of meningeal tumour infiltration in order to identify the optic nerve intradurally and incising the dural cuff of the optic nerve for a short distance, whenever there is evidence of tumour ingrowth. If protrusion of the tuberculum above the line connecting the surface of the nerves as they enter the optic canals is evident, it has to be removed in order to enlarge the transfrontal operative field.
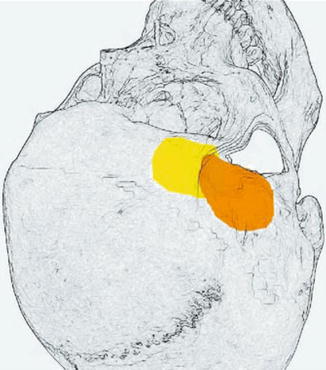

Fig. 17.3
Approaches to SWM: frontolateral (yellow) and standard pterional (orange)
Generally, great caution has to be taken when approaching meningiomas of the medial sphenoid wing because the carotid and middle cerebral arteries may be embedded in the tumour. The dura is opened in a trap-door fashion, and the tumour is approached through the sylvian fissure. The tumour is visualized laterally from above and a plane of dissection is established between the tumour and the frontal and temporal lobe. The tumour capsule is identified and coagulated. The capsule is then opened, and the tumour is debulked by piecemeal resection with the aid of bipolar coagulation, microscissors and ultrasonic aspiration. After the tumour has been debulked and branches of the middle cerebral arteries are encountered, the tumour capsule is carefully dissected off the branches of the middle cerebral artery. The tumour resection is continued along these branches proximally to reach the internal carotid artery (ICA), which may be completely embedded in the tumour. In that case, the vessel is microsurgically dissected free of the tumour. Usually, an arachnoidal membrane separates the tumour from the adventitia of the vessels, and microsurgical dissection can be performed without injury of the arteries. Microsurgical dissection along cerebral arteries may lead to visible vasospasm. In this case, local administration of nimodipine or papaverine sponges can be applied to prevent cerebral ischaemia. However, a systematic analysis concerning the effect of local vasodilator application on postoperative ischaemic events has not yet been performed.
After resection of the tumour, the dural attachment is resected, including hyperostotic bone of the lesser sphenoid wing or the anterior clinoidal process. In tumours involving the cavernous sinus (group 2), the intracavernous part of the tumour is not radically removed to prevent any new cranial neuropathies. Use of the endoscope in terms of an endoscope-assisted technique may be helpful for inspection and removal of hidden remnants [16]. In cases where only the lateral or superior sinus walls are involved, the wall was peeled off and carefully coagulated. After tumour removal, the dura is reconstructed with fascial grafts. In some cases, a muscle graft is required at the cranial base, augmented with fibrin glue, to achieve as watertight a closure as possible. In some patients, temporary postoperative lumbar drainage may be necessary to prevent a cerebrospinal fluid fistula. Bone flaps are repositioned after drilling of hyperostotic bone from the meningioma. Further reconstruction of the orbital roof is not necessary.
The extent of tumour resection is classified according to the Simpson classification [17]: grade I (total tumour resection with excision of infiltrated dura), grade II (total tumour resection and coagulation of dural attachments), grade III (gross total tumour resection without excising dural attachments or extradural extensions (e.g. infiltrated sinus or bone)) and grade IV (subtotal tumour resection).
17.6 Optic Canal Involvement
A special issue in medial SWM is the tumour involvement of the optic canal (Fig. 17.4). MR imaging not always demonstrates the relationship between meningioma and optic canal. In our series of 58 parasellar meningiomas, standard MRI showed tumour growth inside the optic canal in 20 cases. In the remaining 38 cases, there was no optic canal involvement according to radiological criteria. However, in 19 cases the suspicion of tumour extension into the canal rose during surgical inspection and consequently the canal was unroofed. Seventeen times meningioma was detected and removed out of the canal. It can be stated that standard MRI does not show reliably intracanalicular meningioma extension. Fat-suppressed series with thin slices of the orbit enhance the reliability, but compared to the intraoperative assessment, we still detected both false-negative and false-positive cases. In our experience there is no ophthalmologic morbidity due to microsurgical unroofing of the optic canal. Extradural decompression of the optic canal with early intradural opening of the dural sheet enclosing the nerve seems to improve the visual outcome in patients with optic canal involvement [18].
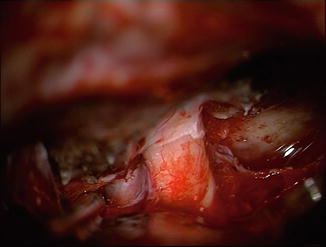

Fig. 17.4
Microscopic view through a frontolateral keyhole craniotomy for a left-sided perisellar meningioma invading the optic canal. Abbreviations: ICA internal carotid artery, M meningioma, OC optic canal, ON optic nerve
17.7 Operative Results
Within the follow-up period (51 patients lost; mean of 63 months; range 2–256 months), clinical and radiological recurrence took place. Figure 17.5 points out how symptoms develop in the postoperative period according to the SWM classification. Recurrence rates were evaluated exclusively for benign meningiomas (Table 17.1) and were compared to patients without osseous involvement (n = 174). There are higher recurrence rates for osseous involved meningiomas (30.5 %) as total removal in these patients is less. Mean time between first treatment and radiological or clinical recurrence was 32 months in WHO I tumours (6–153 months) and 12 months in WHO II tumours (6–18 months). The course of symptoms in the long-term follow-up is exemplarily demonstrated in group VII SWM (Table 17.2): Despite radical removal of infiltrated sphenoid bone, the cardinal symptoms, visual function and proptosis, recurred after 31.1 and 36.1 months, respectively. Comparing the microsurgical results of groups 1 and 2 SWM, significant differences in resection rates according to the Simpson classification and a higher morbidity in visual and oculomotor outcome summing up in a lower Karnofsky index for group 2 SWM have been seen (Fig. 17.6).
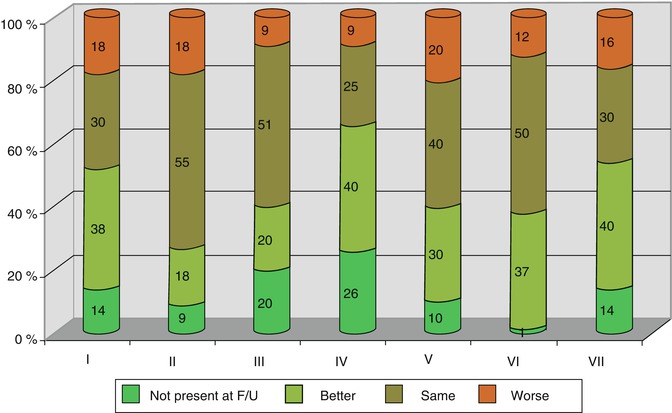
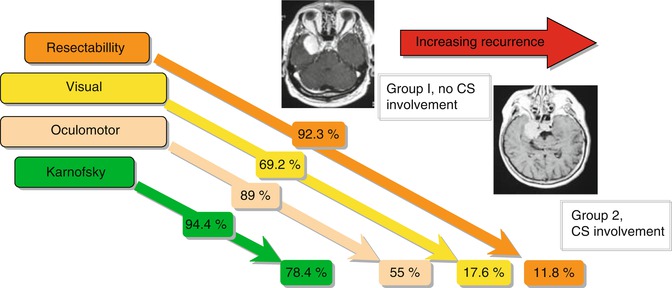

Fig. 17.5
Alteration of clinical symptoms of SWM in the follow-up (F/U) period according to group classification
Table 17.1
Recurrence behaviour of sphenoid wing meningiomas with osseous involvement, compared to SWM without bone infiltration; World Health Organization group I meningiomas
Recurrence | Osseous involved SWM | SWM without osseous involvement |
|---|---|---|
Number of patients | 82 | 174 |
Number of recurrences | 25 (30.5 %) | 20 (11.6 %) |
Grade of resection | ||
Simpson I + II | 38 % | 46 % |
Simpson III + IV | 62 % | 52 % |
Mean time of recurrence (in months) | 32.2 (8–153) | 33.8 (5–86) |
Table 17.2
Clinical course in group VII sphenoid wing meningiomas
Symptoms | Preoperative (%) | Initial post-operative (%) | Long-term outcome (%) | Time to progress (months) |
|---|---|---|---|---|
Visual loss | 41 | 27 | 41 | 31.1 |
Headache | 32 | 9 | 14 | 56.8 |
Proptosis | 91 | 27 | 36 | 36.1 |
Orbital pain | 23 | 23 | 5 | |
Oculomotor palsy | 23 | 23 | 9 |

Fig. 17.6
Comparison of post-operative results in SWM groups I and II. CS cavernous sinus
17.7.1 Radiosurgical Aspects
The primary treatment for meningiomas is microsurgical. In case of high-risk patients or recurrence, however, stereotactic radiosurgery is a valuable option [19]. In a large series overlooking 3,854 meningioma patients treated with radiosurgery by Santacroce et al., morbidity occurred in 10.7 % of cavernous sinus, sellar and middle cranial fossa cases [20]. Interestingly skull base meningiomas were better controlled in this series than convexity meningiomas [20]. Tumour control rates of radiosurgery are definitely promising [7, 20]. Stereotactic radiosurgery may be applied with good success also for meningiomas involving the anterior visual pathways [21].
17.8 Discussion
The heterogeneity among patients with SWM has to be emphasized. Osseous involvement is not restricted to a special group of SWM (Fig. 17.1). Hyperostosis or intraosseous tumour masses can occur in all kinds of SWM, whether they are of globoid shape or en plaque, or whether they were lateral or invading the cavernous sinus (CS). Additionally, the impact of whether the SWM infiltrates the CS or not is of great importance for the approach of the neurosurgeon.
The cause of associated hyperostosis in meningiomas at the sphenoid bone remains a point of controversy – specifically regarding whether this represents a secondary change of the bone without tumour invasion versus direct infiltration of the bone by tumour [22]. Tumours of this type, which invade through the tough fibrous dura and meander through the marrow spaces, are nevertheless unable to penetrate the delicate fibrous tissue of the arachnoid and the pia and thus do not invade the brain. The mechanisms by which meningiomas accomplish this extensive invasion of bone are not at all clear: Preceding trauma, vascular disturbances, enzymatic reactions or stimulation of osteoblasts have been made responsible for the production of bone by tumour or invasion of tumour in the bone [4, 8–10, 23]. Furthermore, it has been demonstrated that hyperostosis is a true formation of additional bone; the invasion is therefore not simply a lytic process. Tumour infiltration of the sphenoid bone has been examined histologically, and proliferation indices has been determined supporting the conclusion by Pompili et al. and Roser et al. that hyperostosis behaves like a slow-growing tumour and must be removed to ensure cure [11, 22]. The surgical impact of a preoperative known bone involvement is high [24]. Radical removal is fairly achievable, especially in en plaque meningiomas or any involvement of the CS. Although, the primary goal should be to completely resect obvious infiltrated bone, when this can be performed with reasonable safety. Additionally the periorbita, which might be infiltrated, should be explored as well [11, 12, 25].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








