Chapter 25 Endoscopic Endonasal Approach for Craniopharyngiomas
Craniopharyngiomas are benign tumors originating from squamous epithelial remnants of Rathke’s pouch, and can arise from any area from nasopharynx to the hypothalamus.1,2 Their consistency can be cystic, solid, or a combination of both; intralesional calcifications are a quite common finding (60% to 80% of cases). They are relatively infrequent, accounting for the 2% to 5% of all intracranial tumors, most frequently involving children (5–14 years) and older adults (50–74 years).3,4 These features contribute to a somewhat unpredictable biological behavior; therefore, the correct management strategy is controversial.5
Surgical management is challenging, especially when complete removal has been advocated as the most effective treatment.6–13 However, despite the benign nature of craniopharyngiomas, complete removal is not always possible due to their proximity and adhesions to vital neurovascular structures. Furthermore, craniopharyngiomas can recur even after radical resection and the surgical removal of recurrent craniopharyngiomas is even more complex due to the scarring and new adhesions.6–12,14,15
The surgical treatment of craniopharyngiomas has been historically performed using various microscopic transcranial approaches, such as the subfrontal, frontolateral, and pterional routes. Trans-sphenoidal approaches, either microsurgical or endoscopic, have been restricted to intrasellar or intrasuprasellar subdiaphragmatic tumors.16,17 Nonetheless, in the last 20 years, the evolution of surgical techniques and technology have led to the progressive reduction of surgical invasiveness along with decreased morbidity. This has resulted in an increase of effectiveness for both transcranial and trans-sphenoidal approaches.18–25
The introduction and diffusion of the extended trans-sphenoidal approach, described by Weiss,26 created a new paradigm in trans-sphenoidal surgery opening a new corridor to the suprasellar space. This extended route for the removal of craniopharyngiomas was successfully adopted by Edward Laws.17,27
The widespread use of the endoscope in sinus surgery was introduced to trans-sphenoidal surgery for the treatment of pituitary tumors.28–30 The wider and panoramic view offered by the endoscope increased the versatility of the trans-sphenoidal approach and permitted its expansion to different parts of the skull base, thus allowing the removal of different “pure” supradiaphragmatic lesions.18,31–45
Furthermore, because craniopharyngiomas are infrachiasmatic midline tumors, the endonasal route provides the advantage of accessing the tumor without the need for brain or optic nerve retraction with a direct visualization through a linear surgical route.46,47
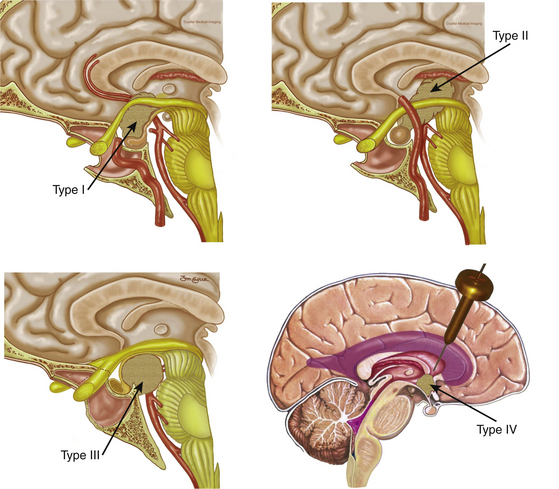
Since the approach has a caudal–cranial orientation, and the tumors are basically infrachiasmatic, the original classification of craniopharyngiomas in relation to the chiasm (pre or post fixed) is not practical. The infundibulum becomes the structure of reference; therefore, craniopharyngiomas have been recently classified accordingly43 as type 1, preinfundibular; type 2, transinfundibular; type 3, post- or retro-infundibular, further subdivision is based on rostral or caudal extension, whether it is to the anterior third ventricular (infundibular recess, hypothalamic) and interpeduncular fossa; and type 4, isolated third ventricular (not well accessed via endonasal routes) (Fig. 25-1). Consequently, specific surgical variations are required for each modality.43
Surgical Technique
Technical Nuances in Craniopharyngioma Surgery
The endoscopic endonasal approach involves the team of two surgeons, using three- or four-hand technique using the visualization provided by rod lens endoscope (18 cm in 4-mm length in diameter; Karl Storz Endoscopy-America, Inc., Culver City, CA) of varying lens angulations (0 degrees and 45 degrees). The surgical team is usually composed of an otolaryngologist and a neurosurgeon experienced in endoscopic endonasal surgery. The otolaryngologist performs the sinonasal approach and then drives the endoscope to provide a dynamic view of the surgical field throughout the procedure, while the neurosurgeon performs a bimanual dissection of the intradural structures.18
Exposure
We customarily initiate the procedure with removal of the inferior half of the right middle turbinate. Thereafter, the most posterior aspect of the posterior ethmoid sinuses is removed to expose the lamina papyracea bilaterally. A Hadad-Bassagasteguy (nasoseptal flap) is raised from the widest nasal cavity (usually the right in view of the middle turbinectomy) using a standard technique (see reconstruction section). At this point, the most posterior 2 cm of the nasal septum are removed and the left middle turbinate is displaced laterally (not resected). A wide sphenoidotomy is opened, enlarging the natural ostium until the lamina papyracea is in plane with the lateral wall of the orbit, the roof of the sphenoid sinus is in plane with that of the nose, and the bottom of the sinus is in full view. All intrasinus septae are removed and, if the sinus is not well pneumatized, the bone is drilled until all surgical landmarks are well exposed. These include the optic nerves and intracranial carotid artery (ICA) canals as well as lateral and medial opticocarotid recesses (MOCR and LOCR, respectively), and the clival recess.48,49 The LOCR lies at the junction of the optic nerve and ICA canals corresponding to the optic strut and the MOCR lies at the confluence of the optic canal and the paraclinoid carotid canal at the lateral portion of the tuberculum sellae. Image guidance is of assistance during these steps of the procedure. It should be remembered that continuous irrigation must be used to avoid thermal injury to the underlying optic nerve. Venous bleeding may be encountered in this region, which also represents the point of insertion of the SIS into the cavernous sinus. The bleeding can be controlled by packing microfibrillar collagen (Avitene, Ethicon, Inc.) or with the use of collagen (Surgifoam, Ethicon, Inc.) mixed with thrombin.
This opens an unobstructed corridor connecting the nasal cavities and the sphenoid sinus; thus, creating a single, large working cavity and bimanual access. The posterior ethmoidal arteries (PEA), encountered 4 to 7 mm anterior to the optic nerve and just anterior to the rostrum of the sphenoid, represent the anterior boundary of the exposure. Staying posterior to the PEAs prevents injury to the cribiform plate and the olfactory system associated with it. The surgical field extends from the posterior ethmoidal arteries rostrally to the clival recess caudally. Subsequently, the endoscope is kept in the patient’s right nostril, slightly stretching it superiorly, over the 12 o’clock position, to create space for the suction tip that is introduced at the 6 o’clock position.
Resection
Above all, it should be kept in mind that the concept of extracapsular dissection introduced by Laws17 takes precedence over any other consideration. One highlight of the endoscopic technique is that it approaches the tumor at its ventral aspect and all of the critical neurovascular structures will lie on its dorsum and perimeter. Furthermore, the endonasal endoscopic technique provides direct visualization of the inferior aspect of the chiasm, the infundibulum, the third ventricle, and/or the retro and parasellar spaces, which can be dissected free from tumor. Debulking of the solid and/or cystic component is performed followed by capsule mobilization and extracapsular sharp dissection. Debulking can be effectively performed using the two-suction technique43 or a combination of a suction and a cut-through instrument. It is important to mention that craniopharyngiomas are often adherent to the chiasm and/or hypothalamus, particularly in cases of recurrence.50 In these circumstances, we advise a partial resection in order to preserve critical neural tissue and its function.
In our experience, we have found that delicate “countertraction,” with a controlled suction fine tip (4 or 6 French), can be very useful to facilitate the isolation of the arachnoid bands and/or the attachments to other neurovascular structures, which then can be sharply dissected,43 as long there is no direct invasion.
Reconstruction
The reconstruction starts with a subdural inlay collagen matrix graft (Duragen, Integra LifeSciences). This graft contains the flow of CSF and facilitates the placement and healing of the graft. Immediately after the inlay reconstruction, the nasoseptal flap is rotated to cover the skull base defect. It is important that osseous surfaces in contact with the flap are stripped of mucosa, which would otherwise prevent adherence and healing of the flap to the defect.51,52 The flap is positioned to cover the defect overlapping its margins to allow contact with bone. In our experience this vascularized flap is superior to any other previously used technique. Its vascularity allows faster healing and an earlier, more resilient seal.
Surgical Considerations Based on Craniopharyngioma Type
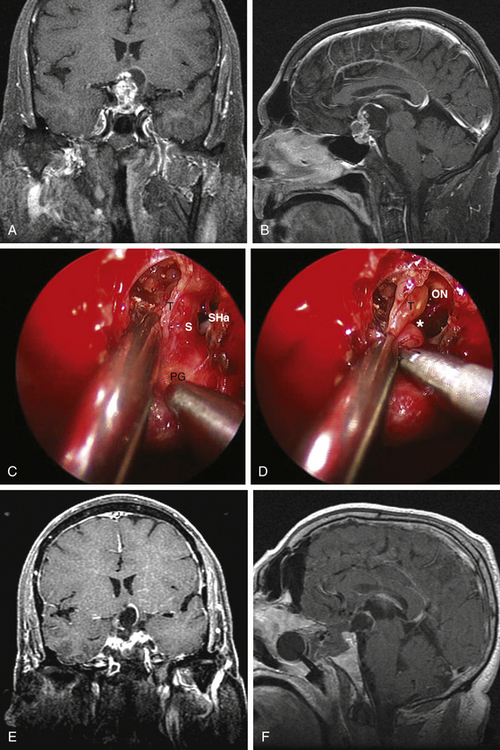
Type 1 craniopharyngiomas are preinfundibular and occupy the suprasellar cistern immediately anterior to the stalk. They tend to displace the chiasm superiorly and posteriorly. Concurrently, they displace the suprasellar arachnoid and the attached superior hypophyseal artery against the retrotubercular dura. These are the most accessible craniopharyngiomas via the endonasal route. Extreme care is critical during the dural opening to preserve the vascular supply for the optic nerves (SHa). Once the SHa are mobilized, the tumor can be debulked and this is followed by extracapsular sharp dissection as described above. SIS ligation and sellar exposure helps in defining the possibility of stalk preservation once the tumor is grossly removed (Fig. 25-2).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree