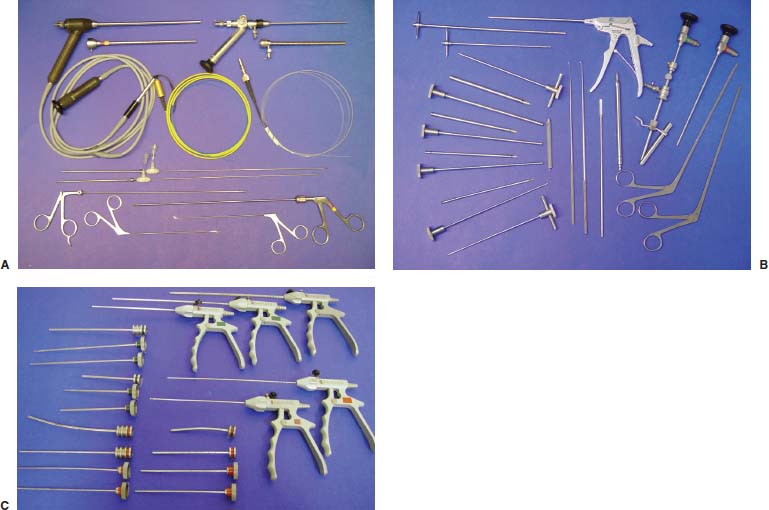
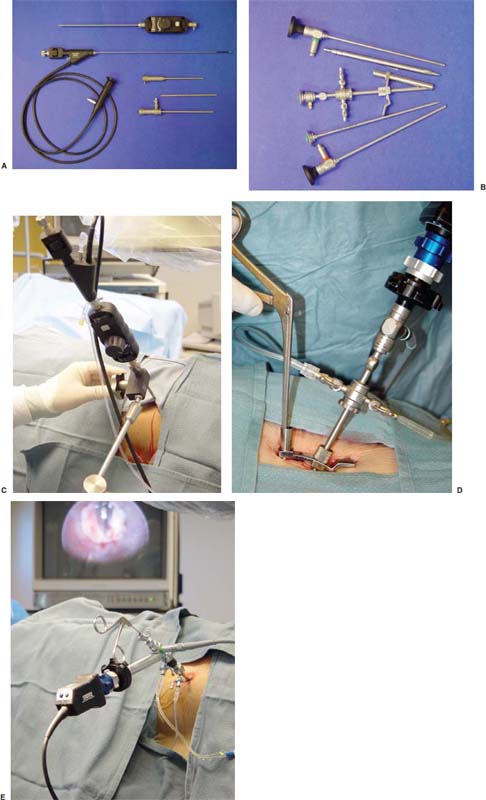
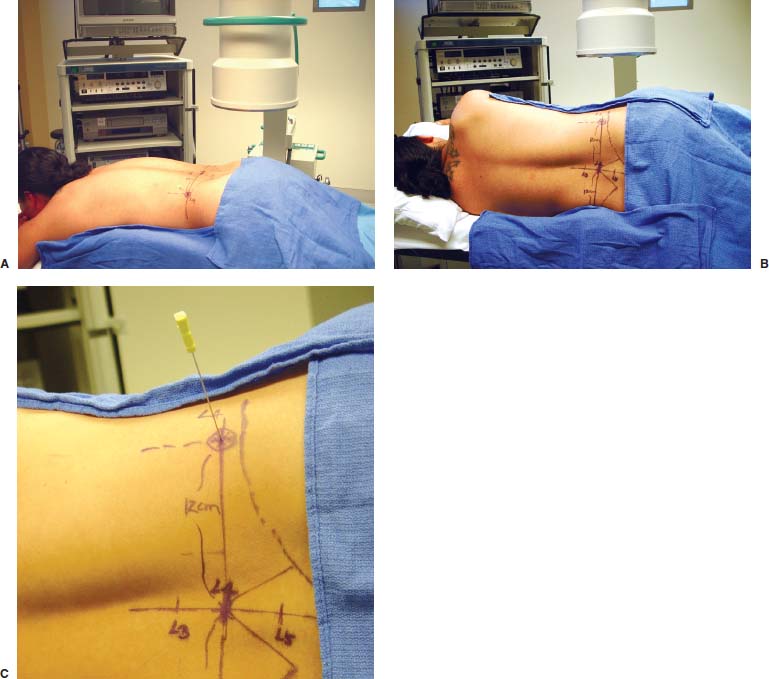
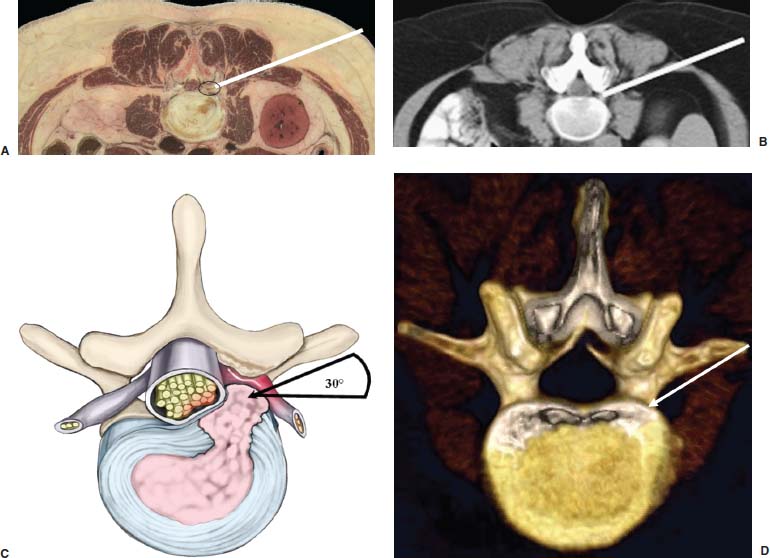
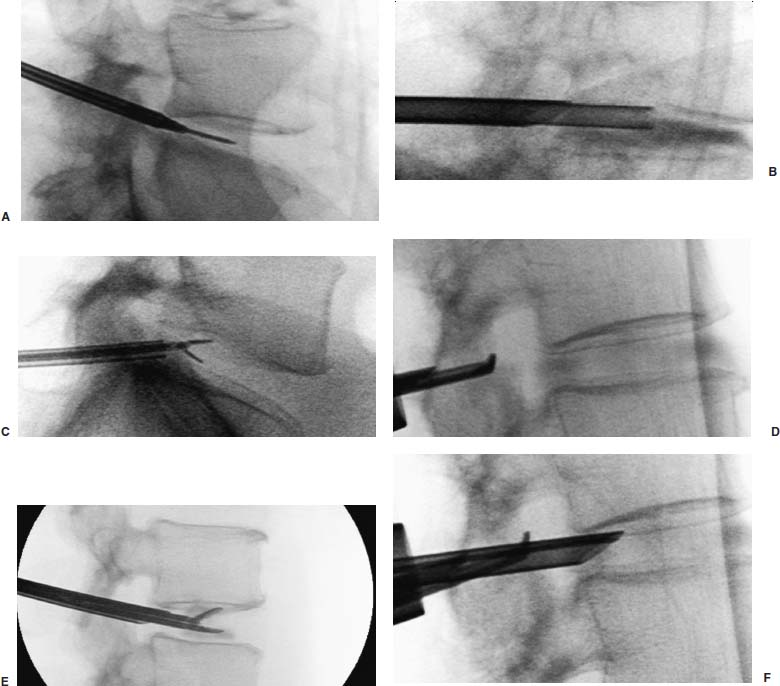
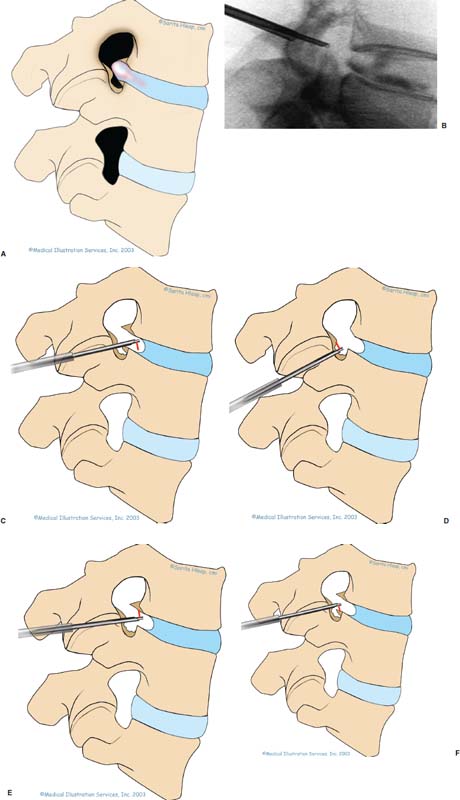
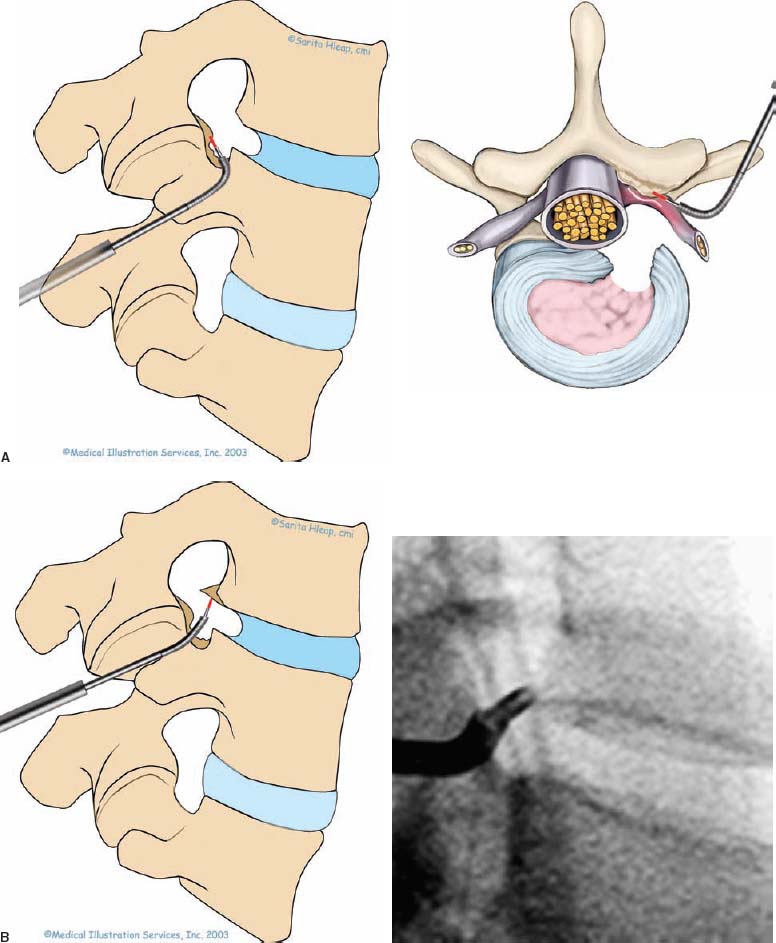
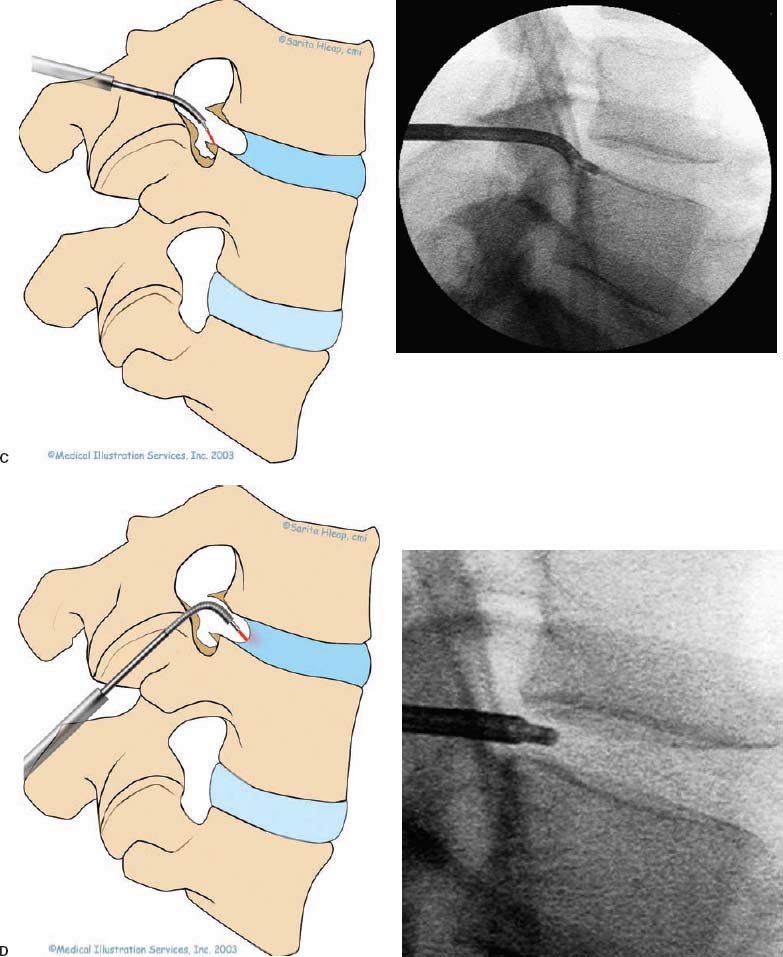
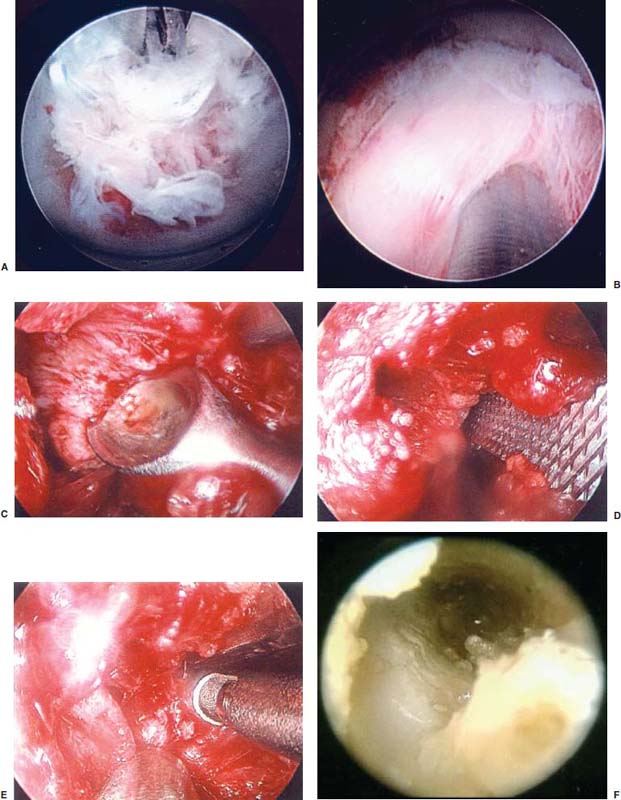
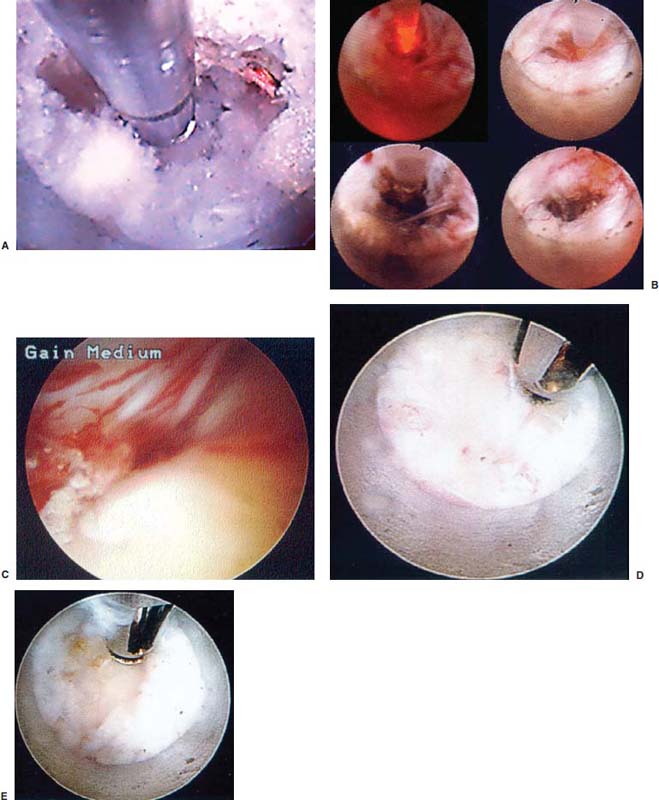
With accumulated experience with endoscopically assisted mechanical and laser lumbar diskectomy,1–18 the need for a more efficient method to decompress the lateral recess19–26 and intervertebral neural foramen from very large or extruded disk protrusions, recurrent disks, scar tissue, and spondylitic spurs became evident. The most frequently seen lumbar spinal disk disease in the elderly is spinal and lateral foraminal stenosis.24,26 Lateral stenosis may be congenital or degenerative when secondary to acute disk disease and spinal trauma. The classic wide posterior decompressive laminectomy with foraminotomy involves extensive muscle and soft tissue dissection for exposure, decompression, and resection of the posterior spinal elements. Despite varying degrees of success,24 it is associated with significant iatrogenic trauma and failed back syndrome.24,26 As a result, the search for a minimally invasive spinal surgery (MISS) technique began. This chapter will describe endoscopic lumbar foraminoplasty and diskectomy, including endoscopic laser foraminoplasty (ELF),10 as reported by Dr. Martin T. N. Knight, with laser application, and minimally invasive transforaminal microdecompressive endoscopic assisted diskectomy and foraminoplasty (TF-MEAD), a new system of more aggressive mechanical instruments and laser application, developed at the California Center for Minimally Invasive Spine Surgery (C-MISS). The pioneering work of Hijikata1 with percutaneous manual diskectomy, Ascher2 and Choy3 with percutaneous laser diskectomy, and the application of endoscopy by Kambin4 and others27–32 resulted in monitored keyhole operations for removal of herniated lumbar disks. The use of endoscopy and laser, especially the holmium side-firing laser, allows removal of large protrusions and extruded disk fragments from the epidural space and stenotic foraminal decompression with ELF, which addresses one level and one side at a time.10,29 This technique takes 90 to 120 minutes to complete using primarily the laser for foraminal decompression and diskectomy.9 Attention is now being directed to treatment of epidural scarring, lateral recess and foraminal stenosis, and advanced degenerative changes that are often bilateral and occur at multiple levels.28 I have developed a more aggressive TF-MEAD system to address endoscopic transforaminal mechanical and laser microdecompressive diskectomy and foraminoplasty in a fast and effective manner for both unilateral single and bilateral multiple levels. These MISS procedures should now join the armamentarium of the spinal surgeon in treating advanced degenerative spinal stenosis and lateral foraminal stenosis. Such procedures require the surgeon to be knowledgeable and competent in MISS, with a thorough knowledge of the procedure of endoscopic lumbar diskectomy and foraminoplasty, pathoanatomy of the neuroforamen and the spine, and the relationships of the lumbar exiting and traversing nerve roots, dorsal root ganglion, facet joint, disks, and vertebrae. Indications Endoscopic lumbar foraminoplasty is indicated in the following clinical situations10,16: Contraindications The endoscopic lumbar foraminoplasty procedure is contraindicated9,15 in the following clinical situations: Instruments and Preparations These surgical instruments are necessary to perform endoscopic laser and/or lumbar foraminoplasty: Anesthesia Knight’s ELF procedure is done under neuroleptic (aware-state) analgesia in the prone position combined with local anesthesia. Patient feedback is essential in these cases when one works around the nerve. TF-MEAD patients are treated in an operating room under local anesthesia and monitored conscious sedation. The anesthesiologist maintains mild sedation, but the patient is able to respond. Two grams Ancef and 8 mg dexamethasone are given intravenously at the start of anesthesia. Surface EEG (SNAP, Nicolet Biomedical, Madison, WI) monitoring provides added precision of anesthesia. Patient Positioning For TF-MEAD, if surgery is unilateral, the patient is placed in a lateral decubitus position (Fig. 19–3B,C), with the painful leg up and both hips and knees in moderate flexion. If the patient has an increased medical risk (i.e., pulmonary, cardiac, morbid obesity, and other high-risk medical conditions), but requires a bilateral procedure, the decubitus position may be used first on one side, then, turning the patient over, on the other side to perform the bilateral procedure in one sitting. Bilateral operations are otherwise performed in the prone position on a radiolucent support similar to the Wilson frame (as for ELF). The arms are supported on arm boards over the head. When local anesthesia and mild sedation are used, the extremities, buttocks, and shoulders are secured and restrained from sudden motion with adhesive tape. FIGURE 19–1 Surgical instruments for endoscopic lumbar foraminoplasty and diskectomy. (A) Percutaneous fiberoptic foraminoscope, 0 degrees, 6 mm OD, 3.9 mm working channel; posterolateral foraminoscope, 6 degrees, 6 mm OD working channel; bare holmium laser fiber, side-firing laser probe (Knight) (Trimedyne), and diskectomy forceps. (B) C-MISS TF-MEAD transforaminal decompressive system: endoscopes (0 and 30 degrees, 4 mm OD,) assisted tubular retractor, trephines, graduated duckbill cannulas, diskectomy rongeur, curette, and 2 mm bone punch. (C) Lumbar diskectomy set with dilators, working cannulas, trephines, and various discectomes. Localization C-arm fluoroscopy is used to identify the lumbar levels relative to the sacrum. The midline, operative levels, and point of entry (operating portal) for surgery are marked on the skin with a marking pen (Figs. 19–3, 19–4A,B). The distance of the point of entry from the midline varies with the height and weight of the patient, but it is ~12 cm at the affected disk level for an averagesize patient. Positioning of the instruments is checked throughout the procedure by fluoroscopy in two planes as often as needed (Figs. 19–5, 19–6B, 19–7B–D). At the involved nerve roots distribution, sterile needle electrodes are placed for continuous intraoperative neurophysiologic EMG monitoring.29 Surgical Technique for Endoscopic Laser Foraminoplasty ELF9,16,28,31 begins with spinal probing and diskography performed at the suspected level, which is clinically appropriate to the site of back or peripheral radiating pain or evidence of clinically related pathology. Knight has described diagnostic spinal probing plus provocative diskogram to identify any concordant painsensitive areas in the lateral recess and foraminal areas in a staged progression of the structures and scar tissue on the disk surface and around the nerve root, the facets, and the walls of the foramen. Symptoms reproduced by spinal probing and diskography determine the extent of the surgical exploration required for the ELF procedure (second stage of ELF procedure), including endoscopic diskectomy, neurolysis, undercutting of facets, osteophytectomy, and/or laser ablation of painful tissue and foraminoplasty (Figs. 19–4A,B, 19–6, 19–9A,D,E). FIGURE 19–2 Steerable spinoscope system, C-MISS TF-MEAD system, and foraminoscope for treatment of disk herniation and foramen stenosis. (A) Steerable spinoscope and cannular set for laser application and with flexible tip. (B) C-MISS TF-MEAD system with a working channel 9.9 mm assisted by endoscopes (0 and 30 degrees, 4 mm OD). (C) Spinoscope surgical application for TF-MEAD. (D) Lumbar foraminoplasty with C-MISS TF-MEAD system in surgical application. (E) Posterolateral foraminoscopy with wide-angle 6-degree, 3 mm working channel operating foraminoscope. The diskography needle is replaced with a guidewire, and a 5 mm dilated tube is inserted into the exit root foramen. The procedure is performed under fluoroscopy. The trocar is then replaced with a working endoscopic channel (Richard Wolf). A side-firing 2 mm internal laser irrigation probe (Fig. 19–1A) is inserted through the endoscope (Trimedyne) to perform ELF (Figs. 19–6, 19–9A,D,E). The extraforaminal zone and margin of the foramen are cleared. The facet joint surfaces are cleaned and undercut to allow the endoscope to enter the epidural space. Vertebral body and facet joint osteophytes, ligamentum flavum and superior foraminal ligaments, and perineuro and epidural scarring are ablated and the facet joint undercut until the anulus and epidural space are visualized. The exiting and traversing nerve roots are mobilized and decompressed medially and laterally until the functional axilla of the root at the apex of the safe working zone is displayed. The nerve is cleared of peripheral fibrosis. Clearance and undercutting are extended along the bone margin to the superior notch with resection of the superior foraminal ligament. Exploration is continued to the inferior pedicle, displacing the anulus and epidural space. Scarring is almost always associated with vascular bands. Inflamed soft tissue on the dorsum of the disk is exquisitely tender in some cases. Osteophytes along the ascending joint of the superior notch, the dorsum of the tibial margin, and the vertebral shoulder are ablated under endoscopic vision by laser. Thermal modulation in conjunction with undercutting of the facet may increase the cross-section of the area of foramen and provide decompression. Endoscopic laser diskectomy and epidural exploration and decompression complete the procedure. Postoperative Care The patient is discharged on the day following surgery. A muscle-balance physiotherapy regimen is recommended on the first day after discharge; later, the program is amplified with neural mobilization drills. Self-help drills are encouraged twice a day thereafter. A pain diary is maintained by the patient to identify the intensity and location of any residual pain. Outcomes Reported are a total of 958 ELF procedures performed on 716 patients who were evaluated at 6 and 12 weeks, 6 months, and yearly following the surgery, unless clinical symptoms required closer supervision. The outcome following ELF remains promising, with 61 to 70% of cases achieving 50% gain in their preoperative Oswestry Disability Index and visual analog pain score. There were 24 complications (1.6%), with diskitis in nine patients, dural tear in one, wound infections in one, foot drop in two, myocardial infarction in one, erectile dysfunction in one, and panic attack in one. Seven percent of patients worsened after the operation. About 3% of patients required subsequent open surgery. Surgical Technique for Transforaminal Microdecompressive Endoscopic Assisted Diskectomy and Foraminoplasty If a pain provocation test and diskogram were not done preoperatively, they are done at the outset. If the diskogram and pain provocation tests are confirmatory, surgery is performed (Figs. 19–2C–E, 19–4, 19–5, 19–7, 19–8, 19–9). An 18-gauge stylet is inserted and incrementally advanced under C-arm fluoroscopic guidance in two planes, at a 60-degree angle from the sagittal plane, targeting toward the center of the disk, through the safety zone, and into the desired interspace. All instrumentation is performed under C-arm fluoroscopic control and endoscopy. The usual procedure for MISS is followed.15 The appropriate-size cannula and dilator are passed over the stylet to the anulus. Under fluoroscopy the extended side of this cannula is turned to face the nerve root in order to retract and protect it. FIGURE 19–4 Safe surgical approach to neuroforamen for endoscopic lumbar foraminoplasty and diskectomy. (A) Posterolateral surgical approach to lumbar disk in cadaveric axial cryomicrotome. (B) Axial illustration of posterolateral lumbar surgical approach for needle placement into intervertebral foramen. (C) Posterolateral surgical approach on prone axial CT image at lumbar disk level. (D) Posterolateral surgical approach into the foramen on axial view of lumbar spine 3D CT image. The cannula retractors have variously shaped extensions like a duckbill (with various lengths, 5 to 10 mm on one side; Figs. 19–1B, 19–5E,F) to retract and protect the nerve root once the cannula is inserted through the foramen into the epidural space and the extension is oriented properly toward the root. The larger, more aggressively toothed trephines (Fig. 19–5B) are then inserted and rotated to cut through anulus, disk protrusion, spur, or spondylitic bar. The cannula is large enough to admit a slim punch (rongeur), spinal disk forceps or pituitary forceps, and full-size curettes to aid decompression of the foramen and the lateral recess (Fig. 19–5C–F). An endoscope can be passed through it instead of the endoscope’s sheath to facilitate mechanical and laser decompression, foraminoplasty, and diskectomy. The endoscope is useful in TF-MEAD surgery for decompression in the lateral recess and periforaminal area. Biting forceps, discectome, and holmium laser with continuous irrigation are used consecutively to perform intradiscal diskectomy; lower energy nonablative laser is applied for shrinking and tightening of the disk (laser thermodiskoplasty).16,17 The decompression area can be enlarged with a larger cannula retractor/trephine set. A small amount of bleeding usually can be controlled with cold saline irrigation and rarely requires hemostasis by laser or bipolar coagulation. Holmium:YAG laser with a side-firing probe or 550-pm holmium laser bare fiber (Fig. 19–1A) is used to ablate the disk and to shrink and contract the disk, reducing the profile of protrusion and hardening the disk tissue (i.e., laser thermodiskoplasty). Disk removal is aided by a rocking excursion of the cannula in a 25-degree arc, a “fan sweep maneuver”16,18 from side to side, that creates an inverted oval coneshaped area of removed disk totaling up to 50 degrees. Laser thermodiskoplasty can also cause sinovertebral neurolysis or denervation. The diskectomies is again used to remove charred debris. FIGURE 19–5 Fluoroscopic view of lumbar endoscopic foraminoplasty and diskectomy with TF-MEAD system. (A) Stylet and dilator in disk space. (B) Trephine for disk and osteophyte decompression. (C) Diskectomy forceps/rongeur for diskectomy. (D) Bone punch for foraminoplasty. (E) Large diskectomy rongeur through duckbill cannula retractor for diskectomy. (F) Duckbill cannula and bone punch in action through TF-MEAD tubular retractor for foraminoplasty. The disk space and neural foramen can be directly visualized and examined by endoscopy to confirm adequate disk decompression and to perform further decompression if necessary. If the foramen is compromised, the depth of insertion of the endoscope is adjusted, the nerve root is again protected by the duckbill extension, and spurs are removed with curettes, bone punches (rongeurs), and Kerrison rongeurs that can be passed through the large cannula for decompressive foraminoplasty, or laser application. An endoscopically assisted larger 9.9 mm tubular retractor system (Figs. 19–1B, 19–2B,D, 19–5B,C,E) has been added to facilitate foraminoplasty (Figs. 19–1B, 19–2B,D, 19–5B,C,E). The steerable Spinescope (Karl Storz) can also be used to perform intradiscal lumbar laser diskectomy and laser foraminoplasty (Figs. 19–2A,C, 19–7, 19–9B). The Spinescope is fixed in a holding device, which allows the surgeon to guide and steer very precisely a flexible fiberscope and a working channel for a laser fiber of 0.6 mm diameter to the pathologic part of the disk and the intra and periforaminal tissue. The laser fiber can be advanced or retracted millimeter by milllimeter inside the disk under direct vision with the fiberoptic endoscopic system, within a given distance. Also, the tip of the applicator/laser fiber can be navigated and angulated from 0 to 90 degrees with fine adjustment and rotated through 360 degrees in all directions. After removing all instruments, 0.25% Marcaine is injected intradermally and into the incision and the paraspinal muscles along the path of the cannulation to prolong analgesia. A bandage is applied at the incision sites. Postoperative Care Ambulation begins immediately after recovery, and the patient is usually discharged 1 hour after surgery. The patient may shower the following day. Applying an ice pack is helpful. NSAIDs are prescribed, and mild analgesics and muscle relaxants are recommended as needed. Patients typically return to usual activities in 10 days to 3 weeks, provided heavy labor and prolonged sitting are not involved. Outcome At C-MISS, the first 60 consecutive cases of the 180- plus cases treated with TF-MEAD to date (since year 2000) included 31 males and 29 females. Thirty-six (60%) had surgery at one level, 24 (40%) at two or more levels, eight bilaterally. All patients had complained of chronic low back pain with radicular pain, usually unilateral, and in eight instances, bilateral. Physical examination, as well as positive MRI and/or CT, EMG, and intraoperative or preoperative diskograms appropriate to the symptomatic levels, confirmed the diagnosis and levels. All had failed to improve after at least 12 weeks of conservative therapy. There were no significant intraoperative or postoperative complications. Fifty-three patients (88.33%) had a good or excellent result (McNabb criteria). Six had some continuing complaints but were improved overall. One did not significantly benefit from the procedure. Most patients found this procedure extremely gratifying. FIGURE 19–7 Fluoroscopic view and illustration of laser side-firing probe in action for lumbar laser foraminoplasty with steerable spinescope. (A) Illustrations of lumbar laser foraminoplasty with spinescope for facet hypertrophy (lateral and axial views). (B) Illustration and fluoroscopic lateral views of lumbar laser foraminoplasty with spinescope for upper shoulder osteophyte. (C) Illustration and fluoroscopic lateral views of lumbar laser foraminoplasty with spinescope for lower shoulder osteophyte. (D) Illustration and fluoroscopic lateral views of lumbar laser diskectomy with spinescope.
19

Endoscopic Lumbar Foraminoplasty

| Stage | Watts | Joules | ||
|---|---|---|---|---|
| First | 15 | 1500 | ||
| Second | 10 | 500 |
*Laser energy used (at 10 Hz) 5 seconds on and 5 seconds off for TF-MEAD.
The TF-MEAD, with mechanical and laser applications using newly devised, more aggressive instruments, allows wider and more complete removal of larger disks and decompressive foraminoplasty at bilateral and multiple levels in one sitting. It has proven to be safe and effective. To become competent and avoid complications, the TF-MEAD or ELF surgeon must have a thorough knowledge of the surgical anatomy and the procedures, and specific surgical training with hands-on experience in a laboratory and working closely with an endoscopic surgeon expert at these procedures through the steep surgical learning curve.
Advantages
With endoscopic lumbar foraminoplasty, decompression of the lateral recess and foramen is accomplished at a single sitting and can be at multiple levels or bilateral. Other advantages are:
- Same-day outpatient procedure
- Less traumatic, both physically and psychologically
- Small incision and less scarring
- Zero mortality
- Minimal blood loss and little or no epidural bleeding
- No dissection of muscle, bone, ligaments, or manipulation of the dural sac or nerve roots
- Does not promote further instability of spinal segments or adjacent segment recurrent disks
- Commonly done under local anesthesia; no general anesthesia necessary
- Multiple level diskectomy feasible and well tolerated28
- Least challenging to medically high-risk patients and the obese
- Exercise programs can begin the same day as surgery
- No significant incidence of infection
- Direct endoscopic visualization and confirmation of the adequacy of decompression
- Minimal use of analgesics postoperatively
- Earlier return to usual activities, including work
- Costs less than conventional lumbar surgery
Disadvantages
Disadvantages of endoscopic lumbar foraminoplasty include:
- More than grade I spondylolisthesis
- Acute traumatic or neoplastic conditions
- ELF unisegmental and unilateral
- ELF requires overnight stay
Complications and Avoidance
A thorough knowledge of the endoscopic lumbar foraminoplasty and diskectomy procedures and surgical anatomy of the lumbar spine and intervertebral foramen, careful selection of patients and preoperative surgical planning with appropriate diagnostic evaluations, and meticulous intraoperative technique facilitate the ELF and TF-MEAD procedures and prevent potential complications. All potential complications of open lumbar disk surgery are possible, but they are much less frequent in endoscopic lumbar foraminoplasty.26,28,31
- Inadequate decompression of disk material: Minimized by using such multiple modalities and instruments as forceps, discectome, and laser application both to vaporize tissue and to perform thermodiskoplasty (shrinking and hardening the disk with laser energy at a lower level).
- Neural injury: Rare with MISS. Nerve root injury, although possible, can be avoided with the warning provided by continuous intraoperative neurophysiologic monitoring (EMG/NCV)31 and direct endoscopic visualization. Operating strictly within the safe zone or triangle minimizes the root’s exposure to injury. Sympathetic nerve injury is extremely remote because the procedure is largely intradiscal or in the foramen. Use of local anesthesia with a verbally responsive patient also provides a further warning system.
- Ganglion (dorsal root) injury: One of the commoner complications reported in posterolateral lumbar percutaneous approaches to the foramen is dysesthesia (the incidence of transient dysesthesia has been reported as high as 25% transient at one center but usually less than 2 to 3%, and permanent less than 1%)31 in the leg on the operated side. Careful technique guided by close endoscopic and C-arm fluoroscopic monitoring and knowledge of the surgical anatomy of the lumbar nerve root, dorsal root ganglion, and foramen minimize this complication.
- Operating on wrong level: A major complication of disk surgery at any level of the spine is operating at the wrong level. Proper use of digital C-arm fluoroscopy for correct anatomical localization avoids operating at the wrong disk level. Routine pain provocation test and diskogram give additional verification of the proper level.
- Infection: Avoided by careful sterile technique, the much smaller incisional area, the absence of prolonged retraction of soft tissues, and the use of prophylactic antibiotics IV intraoperatively.
- Diskitis: Prophylactic antibiotics, continuous irrigation of the interspace throughout the procedure, and the introduction of instruments through a cannula without contact with the skin tissues help minimize the incidence of infectious diskitis.
- Aseptic diskitis can be prevented by aiming the laser beam in a “bowtie” fashion to avoid damaging the end plates (at 6 and 12 o’clock).
- Hematoma (subcutaneous and deep): May occur with MISS (reported in the early literature), but is minimized by careful technique; by not prescribing anticoagulants, aspirin, or NSAIDs within a week prior to surgery; by doing a basic clotting screening preoperatively; by application of gentle digital pressure or placing a full IV bag over the operative site for the first 5 minutes after surgery; and by application of an ice bag thereafter.
- Vascular injuries: They are extremely rare when care is taken to remain within the disk space with stylets and cannulas. The aorta, vena cava, femoral arteries, and veins are best avoided by accurate placement of all instruments. No vascular injury has been reported with lumbar MISS since the early experience with similar procedures.
- Bowel and ureteral injuries: Ureteral injuries have not been reported with MISS. Bowel perforation was reported in the early experience, but was not reported in a multicenter study31 of more than 26,860 cases.
- Cerebrospinal fluid leak or dural injury: Dural injury has not occurred in any sense other than as evidenced by spinal headache and presumed CSF leakage. The incidence of only transient leakage in the multicenter study32 was less than 1%, and none required surgery to repair a dural tear. Spinal headache has responded to simple blood patches.
- Excessive sedation: Avoided by surface EEG monitoring, providing more precise estimation of the depth of anesthesia; reduces amount of anesthetics and prevents excessive or insufficient sedation. Operations under local anesthesia with conscious sedation allow the patient’s responsiveness to be directly tested.
- Soft tissue injuries due to prolonged forceful retraction as occurs in many open disk operations are not an issue with TF-MEAD or ELF.
- Operating on wrong level: A major complication of disk surgery at any level of the spine is operating at the wrong level. Proper use of digital C-arm fluoroscopy for correct anatomical localization avoids operating at the wrong disk level. Routine pain provocation test and diskogram give additional verification of the proper level.
Case Illustration
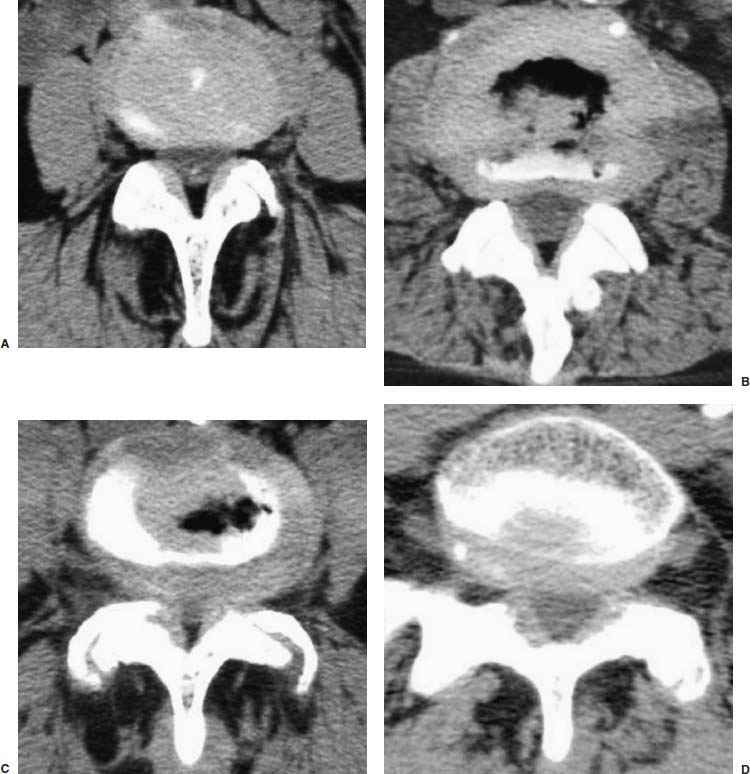
An 80-year-old man was evaluated for complaints of progressive low back and right, more than left, lower extremity pain with numbness and tingling of both legs intermittently for 3 to 4 years, with neurogenic claudication after walking for one or two blocks in both lower extremities. After evaluation in his home country, he was treated with analgesics and an anti-inflammatory medication for arthritis of the spine, along with pain injectional therapy. He had paraspinal muscle spasm and tenderness with limited range of motion of the back. Deep tendon reflexes were decreased for right knee and both ankle jerks; strength and gait were normal. Straight leg raising was 70 degrees on the right and 75 degrees on the left. Pain and touch sensation were impaired on the dorsal and lateral aspect of the right foot and ankle. EMG showed bilateral L5 and S1 radiculopathy; CT image demonstrated disk defects of 3 mm at L1–L2, 4 to 5 mm at L3–L4, L4–L5, and L5–S1 with bilateral foraminal stenosis (Fig. 19–10). He had facet joint hypertrophy and intervertebral spurring. The patient was positive on provocative diskogram at all four levels. Outpatient TF-MEAD surgery was performed at four levels bilaterally. The patient was ambulatory postoperatively, with relief of all leg pain and numbness.
Endoscopic lumbar decompressive diskectomy and foraminoplasty (including ELF and TF-MEAD) has replaced open decompressive lumbar surgery for lateral spinal stenosis and disk herniation in this group of treated patients and has proven to be safe, less traumatic, easier, and efficacious, with significant economic savings. Both ELF and TF-MEAD are minimally invasive techniques that decrease intraoperative and postoperative complications significantly by using endoscopic surgical techniques.
ELF is a technically demanding procedure that requires 90 to 120 minutes to affect once the learning curve has been surmounted. It is intended for and ideally applied to the treatment of unilateral and unisegmental pathology; however, the complication rate of ELF has been significantly lower than conventional spinal surgery. With this procedure, MISS is no longer confined to percutaneous endoscopic diskectomy but also deals with effective decompression of lateral spinal stenosis, lateral recess, and foraminal large extruded herniated disks effectively.
TF-MEAD combines more aggressive mechanical decompression and laser application effectively to treat spinal pathology at multiple levels and bilaterally. Many elderly patients (even octogenarians and beyond) suffering symptoms caused by lateral spinal stenosis and disk problems can be successfully treated. The results of this operation can be extremely gratifying for both the patient and the surgeon.
These procedures require a knowledgeable and competent surgeon with a thorough appreciation of the surgical anatomy. A minimally invasive spine surgeon must have specific surgical training with hands-on experience in the laboratory and, most importantly, must spend time working through the steep surgical learning curve with an endoscopic spinal surgeon expert at this procedure.
REFERENCES
2. Ascher PW. Application of the laser in neurosurgery. Laser Surg Med. 1986;2:91–97.
19. Malis LI. Instrumentation and techniques in microsurgery. Clin Neurosurg. 1979;26:626–636.
< div class='tao-gold-member'>









