18 Endoscopic Management of Anterior Skull Base Meningoencephalocele
Daniel Timperley, Rodney J. Schlosser, and Richard J. Harvey
 Introduction
Introduction
Anterior skull base meningoencephaloceles are herniations of brain and meningeal tissue into the nose or paranasal sinuses. There has been an evolution in management of these lesions from open to transnasal endoscopic approaches. The endoscopic approach is effective1–3 with the potential for lower morbidity4 and shorter hospital stay5 than the traditional open approaches.
 Etiology
Etiology
Meningoencephaloceles may be congenital or acquired as a result of increased intracranial pressure (ICP) or surgical or accidental trauma.
Congenital
Frontoethmoidal meningoencephaloceles originate between the frontal and ethmoid bones at or anterior to the foramen cecum and present externally on the face from the glabella to the columella, often in association with nasal dermoid cysts. Basal types present intranasally and may occur in any location, with 14% of ethmoid bones demonstrating bony dehiscences in anatomic studies.6 The most common sites clinically have been reported as the cribriform adjacent to the middle turbinate attachment and from the superior and lateral walls of the sphenoid (Fig. 18.1).6,7 Transsphenoidal encephaloceles are associated with other midline abnormalities.6,8 The optimum timing of repair has yet to be determined and is a balance between growth of the child to improve surgical access and the risk of meningitis or cosmetic deformity. The risk of meningitis before the age of 5 years in the absence of a cerebrospinal fluid (CSF) leak seems to be low, so the indications for early repair are cosmetic deformity or CSF leak, and successful endoscopic repair has been reported in a 23-month-old.9
Increased Intracranial Pressure and Benign Intracranial Hypertension
The majority of spontaneous or idiopathic meningoencephaloceles are associated with evidence of increased ICP and are now thought to be a clinical manifestation of raised intracranial hypertension on a spectrum from benign intracranial hypertension (BIH) to pseudotumor cerebri.10–12
Patients with BIH are typically obese middle-aged women, and they may present with headache, pulsatile tinnitus, balance problems, and visual disturbance. However, in the presence of an active CSF leak, ICP may be artificially lowered. These symptoms may only manifest following closure of the leak with a subsequent increase in ICP.12–14
The postulated mechanism is erosion of the skull base at points of inherent structural weakness due to constant pulsatile pressure.12 Common sites for spontaneous encephaloceles are the cribriform plate and the lateral pneumatized sphenoid recess. Those in the lateral sphenoid recess typically occur lateral to the foramen rotundum (Fig. 18.2) and vidian canal in patients with extensive pneumatization of the sphenoid.15 Some authors have described these lesions as occurring though the lateral craniopharyngeal (Sternberg’s) canal, but this was described posteromedial to the foramen rotundum and it is argued that the skull base defect is due to the effects of increased ICP on a thin skull base.12,16
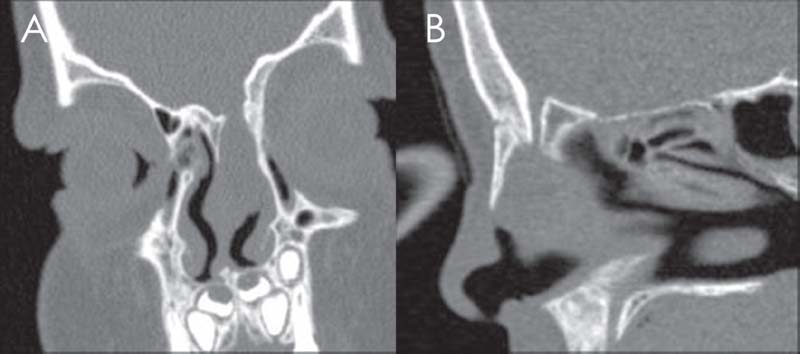
Fig. 18.1 Congenital meningoencephalocele of the right fonticulus frontalis defect type. (A) Coronal view. (B) Sagittal view.
 Diagnostic Workup
Diagnostic Workup
Laboratory Investigations
In patients who present with a suspicion of CSF rhinorrhea, the diagnosis should be confirmed by testing a sample of the nasal discharge. β2-transferrin is formed by desialization of β1-transferrin by cerebral neuraminidase in CSF. It is found only in CSF, vitreous humor, and perilymph, and testing can be performed on as little as 0.5 mL of fluid.17
β-trace protein is prostaglandin D synthase found in CSF and is an alternative marker for CSF, which has been used mainly in parts of Europe. It requires a larger sample than β2-transferrin but has a lower processing cost, and the assay is more rapid (15 to 20 minutes).13 In those with spontaneous leaks, a lumbar puncture for opening pressures (normal range, 5 to 195 mm H2O)18) may be useful preoperatively to guide perioperative management. However, several large series has shown excellent results with only postoperative management of increased ICP.1,12,19
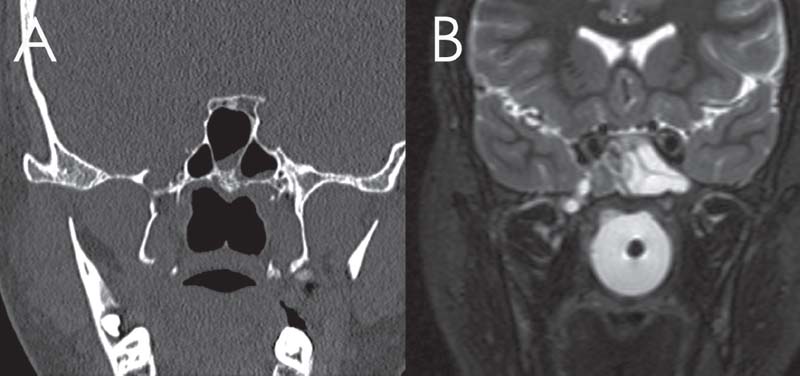
Fig. 18.2 Pits and small diverticulum within the right V2 and foramen rotundum. (A) Computed tomography (CT). (B) T2-weighted magnetic resonance imaging (MRI).
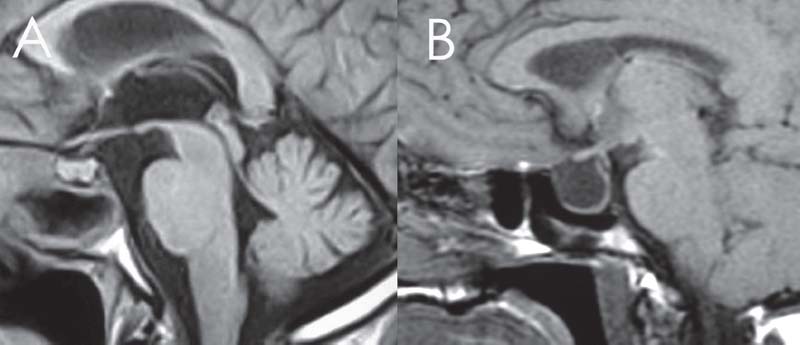
Fig. 18.3 The normal sella (A) and the empty sella (B) on T1-weighted MRI.
Imaging
Coronal, axial, and parasagittal bone window computed tomography (CT) images of the paranasal sinuses and skull base are essential in defining the bony defect and the anatomy for surgery, but cannot distinguish the encephalocele from nasal polyp or secretions.
Magnetic resonance imaging (MRI) and MRI cisternography (using highly T2-weighted images without intrathecal contrast) are useful in determining the contents of the sac, differentiating the sinus mucosa and mucus from the meningoencephalocele and demonstrating signs of increased ICP.20 Radiographic evidence of increased ICP include a totally or partially empty sella; optic complex abnormalities; dural ectasia, most commonly at Meckel’s cave; and arachnoid pits, which are small pits in the skull base hypothesized to occur at the site of arachnoid granulations due to increased CSF pressure (Figs. 18.2 and 18.3). The optic complex abnormalities are vertical tortuosity of the optic nerve, increased subarachnoid space around the optic nerve (total width of CSF greater than the diameter of the nerve), and scleral flattening. Meningoencephalocele formation, especially multiple (Fig. 18.4), in itself is considered a sign of BIH.11,12,14,21 CT angiography or magnetic resonance angiography (MRA) may be used, particularly in large lesions to show vessels within the sac (Fig. 18.5). CT cisternography can help define the meningoencephalocele and can show the site of an active CSF leak when there is the need for further diagnostic information (Fig. 18.6).20,21
 Endoscopic Approach
Endoscopic Approach
Indications and Advantages
Repair is indicated to separate the cranial and nasal cavities. The rate of intracranial complications in nonleaking meningoencephaloceles is unknown. However if there is an associated CSF leak, the risk is high, with 13.9 to 41% of patients reported to have had an intracranial complication in different series, dropping to <1% following successful repair.1 The endoscopic approach allows treatment of the meningoencephalocele without brain retraction or external incisions. Olfaction is usually preserved and sequelae of brain retraction such as postoperative epilepsy avoided.
Contraindications
Active acute or subacute bacterial sinusitis is the main contraindication and should be treated prior to repair. Chronic inflammatory sinus changes do not prevent successful closure, but management should be optimized prior to surgery. Lesions in the lateral frontal sinus (lateral to the medial orbital wall) or supraorbital ethmoid cells can be difficult to access transnasally and may require an ancillary frontal trephine or an open approach. The lack of a multidisciplinary team and specialized equipment should be considered a contraindication.
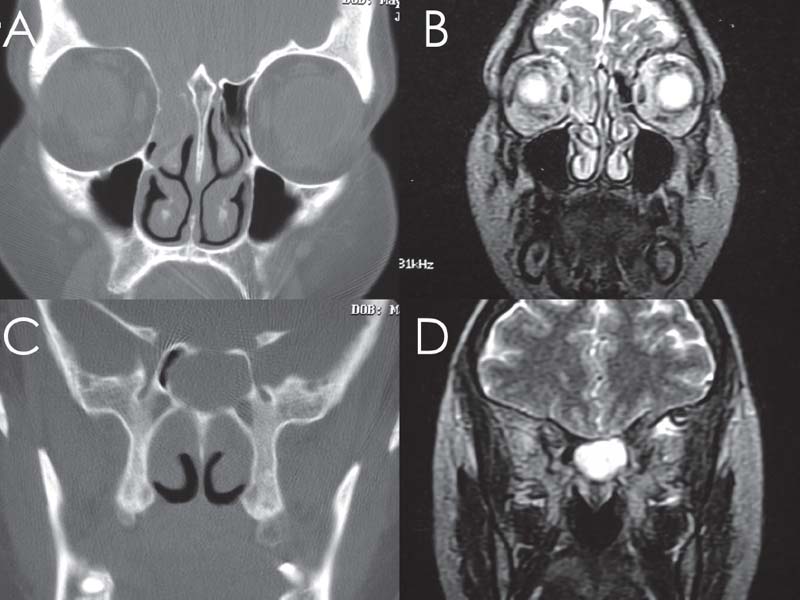
Fig. 18.4 Multiple meningoencephaloceles are a hallmark for benign intracranial hypertension. CT (A,C) and MRI (B,D) of right cribriform defect in the same patient with a sphenoid defect.
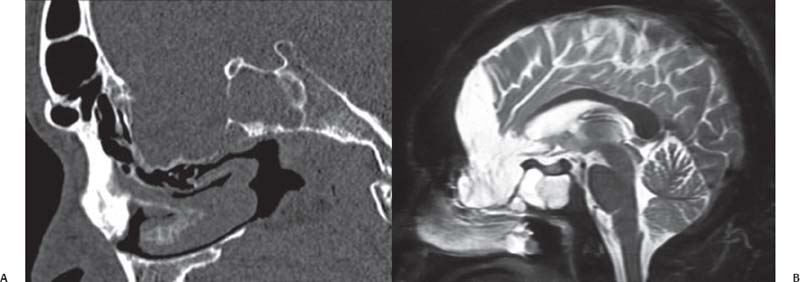
Fig. 18.5 Large vascular loop may be part of the sac contents for very large defects and should be evaluated with both CT (A) and MRI (B).
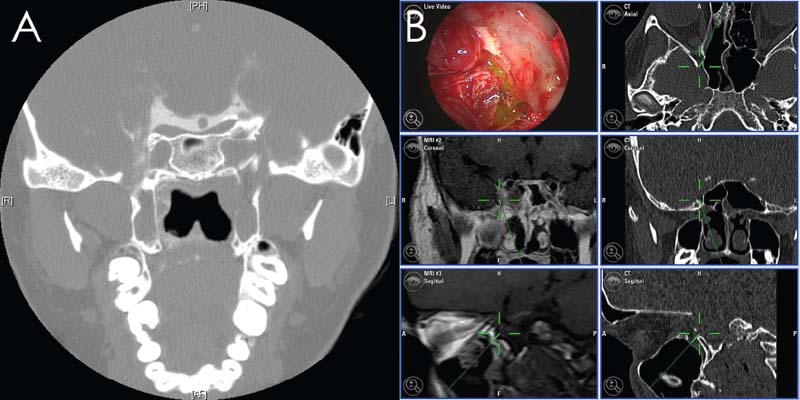
Fig. 18.6 Contrast cisternography (A) and intrathecal fluorescein (B) can be very helpful in identifying unusual cerebrospinal fluid (CSF) leaks. In this patient, contrast and CSF leaked into the nasopharynx from a right foramen rotundum defect.
 Surgery
Surgery
Instrumentation
A standard endoscopic setup is required. The bipolar forceps and frontal instruments (when required) are the essential additions to this setup. Image guidance systems, although not essential in every case, are extremely useful as the approach becomes more complex.
Operative Setup
The patient is placed supine in the reverse Trendelenburg position with the head turned slightly toward the surgeon. Intravenous antibiotic such as cefazolin is given at induction of anesthesia. Total intravenous hypotensive, bradycardic anesthesia is preferred to reduce bleeding, typically with remifentanil and propofol infusions.22,23 Topical epinephrine 1:1000 is applied on cottonoids for 10 minutes before the start of the procedure, but ceased once dura is reached.
A lumbar cistern catheter enables measurement of CSF opening pressure, instillation of fluorescein, and postoperative lumbar drainage if required. Intrathecal fluorescein in the setting of an encephalocele can be useful in identifying a difficult leak site (Fig. 18.6), confirming closure of the CSF leak, and showing other leaks if multiple defects are present (Fig. 18.4). A dose of 0.1 mL of 10% fluorescein diluted into 10 mL of CSF instilled over 10 minutes is safe, although not approved by the Food and Drug Administration (FDA).21,24,25
Approach
Prior to beginning the approach, the nature of the planned repair should be determined so that local flaps can be preserved if necessary.
Frontal Sinus
A frontal recess approach may be sufficient or a modified endoscopic Lothrop procedure may be required both to gain access for the repair and to ensure adequate frontal sinus drainage postoperatively to prevent subsequent mucocele formation. For frontal sinus defects lateral to the medial orbital wall, a frontal trephine may be required as an adjunctive procedure, or an osteoplastic flap may be required.26 Obliteration and ablative approaches should be avoided as the risk of long-term sequelae is very high. The potential for creating a mucocele in this setting is significant.27,28 This may sentence a patient to a life of chronic frontal sinusitis, headaches, intracranial complications, and cosmetic deformities of the forehead. Unfortunately, these iatrogenic conditions only reveal themselves 8 to 10 years29 after the obliteration. Where possible, maintenance of frontal sinus ventilation should be performed. Defects involving the frontal sinus can be classified into three important management groups based on anatomic localization that impacts the surgical approach (Table 18.1).26
| • Type A | Defects wholly within the frontal sinus (medial or lateral to the lamina papyracea) |
| • Type B | Defects that involve any part of the frontal recess |
| • Type C | Defect not within the frontal recess but anatomically adjacent to the frontal recess. |
Ethmoid Roof/Cribriform Plate
An ethmoidectomy provides access to the ethmoid roof and lateral lamella, and, combined with middle turbinate resection, the cribriform plate. If the middle turbinate is resected, it should be taken as high as possible to enable the graft to sit flat on the skull base and to prevent lateralization and frontal recess obstruction. Typically, the cribriform bone is very thin and fragmented. It is common to create a final bony defect much greater than the original neck of the meningoencephalocele to define the boundaries of the repair.
Sphenoid
Lesions located close to the midline can be addressed using a transnasal or transethmoidal approach with a wide sphenoidotomy, with or without posterior septectomy and removal of the intersinus septum. The lateral recess usually requires a transpterygoid approach (Fig. 18.7). An ethmoidectomy, removal of the entire front face of the sphenoid, and a wide middle meatal antrostomy are performed and the sphenopalatine foramen identified. The posterior wall of the maxillary sinus is removed, exposing the pterygopalatine and infratemporal fossa. The maxillary artery and its branches are identified, clipped, and divided or transposed inferiorly to allow access to the pterygoid root. The remaining front face of the sphenoid laterally is removed to expose the defect. Care is taken to preserve the maxillary nerve, pterygopalatine ganglion, and branches, particularly the descending palatine nerves, which are at risk during this approach. The vidian nerve and its parasympathetic fibers that join V2 are preserved if possible to prevent the risk of postoperative dry eye.11,30,31
Resection and Preparation
The encephalocele is carefully resected back to the skull base defect using bipolar diathermy. Alternatively, the sac can be opened and the defect defined from within. The surrounding mucoperiosteum is removed to prepare the graft site. In the cribriform plate, this is often not possible, and the mucosa is instead ablated.15 The ascending olfactory fascicles prevent simple stripping of the mucosa. If a lumbar drain is used, removal of 10 to 15 mL of CSF with an ongoing drainage of 5 to 10 mL/hour can facilitate reduction of the encephalocele intracranially.13,21 Most underlay grafts are subdural, but the edges of the dura can be carefully elevated for a few millimeters around the defect if an intracranial extradural layer is required.
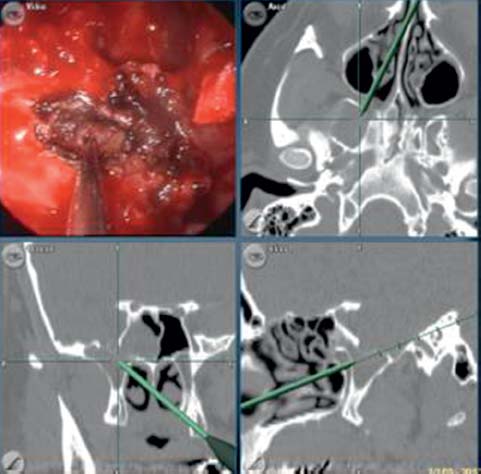
Fig. 18.7 Very lateral sphenoid defects are often best repaired via a transptyergoid approach. This access takes a direct route through the back of the maxillary sinus rather than using angled endoscopes and instruments via a sphenoidotomy.





