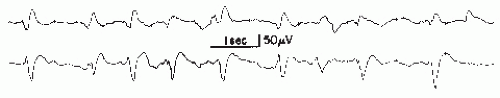Epilepsy in Adults and the Elderly
Bernard S. Chang
Donald L. Schomer
Ernst Niedermeyer
INTRODUCTION
It is difficult to imagine the practice of modern epileptology without the availability of electroencephalography (EEG), in all of its various forms. The two fields have been closely intertwined over the past decades, the latter being the objective accompaniment to the clinical art of the former. As some epileptologists have remarked, “one feels that one knows a patient a little better after seeing his EEG.” While electroencephalography (EEG) clearly has many other important uses in neurologic practice (detailed throughout this volume), it has revolutionized the entire field of epileptology and continues to do so with each technical advance.
This chapter defines and describes the various EEG abnormalities seen in patients with epilepsy, including both those that occur during seizures and those that occur between seizures, as well as similar EEG findings that have less certain significance and relationship to seizure disorders.
PRINCIPAL DIFFERENCES BETWEEN INTERICTAL AND ICTAL DISCHARGES
Naturally, one would like a distinction to be made between ictal and interictal paroxysmal activities. This logical differentiation, however, is beset with great difficulties. It is sometimes difficult or even impossible to distinguish between ictal-clinical, ictal-subclinical, and interictal paroxysmal EEG activities, even with the use of neuropsychologic tests. We must acknowledge the existence of a gray zone between interictal and ictal paroxysmal activities.
Sudden Change of Frequency
There are valid guidelines for the detection of true ictal events. The onset of a clinical seizure may be characterized by a sudden change of frequency. A new type of rhythm appears, hesitantly, and then more distinctly; soon it boldly dominates the tracing. This rhythm may be in alpha frequency or it may be faster or slower; it clearly demonstrates a new element of the tracing, indicative of a completely new electrophysiologic event. The abnormal rhythm may or may not show spiky character. It tends to become slower with increasing amplitudes and more distinct spiky phases of the rhythmic waves.
Sudden Loss of Voltage
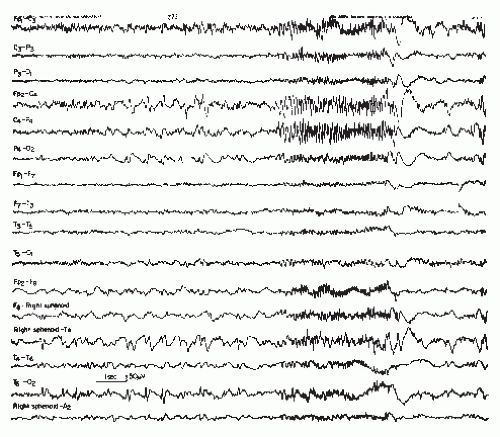
Sudden “desynchronization” of electrical activity is found in electrodecremental seizures. The onset of these attacks may look almost flat locally and/or diffusely, but extremely fast very low voltage activity may gradually increase in voltage with decreasing frequencies. Ictal rhythmic activity may soon become preponderant, similar to that found in the sudden change of frequency. Arroyo et al. (1) have stressed the role of the frontal lobes in electrodecremental seizures. Electrodecremental seizure represents the most common EEG expression of infantile spasms occurring in the West syndrome (2,3).
Sudden Increase of Voltage
The classical example is the sudden steep rise of amplitude in a classical petit mal absence with 3/sec spike waves. There is hence considerable variability in the ictal EEG events. This has been quite discouraging for those concentrating on automatic devices for seizure detection. Even the eye of the experienced electroencephalographer may have difficulties in the determination of ictal episodes.
Ultraslow and Ultrafast Frequencies
These two aspects of paroxysmal activity are playing an increasing role in our understanding of epileptiform EEG activity, and are discussed separately within this volume.
Problems of Terminology
Definitions of EEG events are indispensable, but they are also quite difficult to arrive at and are often unsatisfactory. Attempts to reassess the EEG terminology have been made from time to time; they often result in neologistic construction of terms that fail to receive general acceptance. The EEG terminology is filled with widely used, popular, and, alas, often quite sloppy and inaccurate terms.
A preliminary proposal by the International Federation of Societies for Electroencephalography and Clinical Neurophysiology (4) was followed by a definitive proposal (5) and a glossary of terms used by clinical electroencephalographers (6). The handbook catalog of Dutertre (7) exudes Cartesian lucidity and deserves admiration. Unfortunately, the definition of electrophysiologic events can never do justice to all facets of the phenomenon.
As to epileptic discharges, there has been considerable disunity in the ranks of electroencephalographers. The term epileptic discharge has been attacked and condemned on the ground that such discharges may occur in the absence of clinical seizure manifestations or in individuals who have never had seizures. The same is true for the widely used term seizure discharge; even paroxysmal discharge, certainly a more cautious and unassuming term, has not found general acceptance.
INTERICTAL EPILEPTIFORM DISCHARGES
Spikes (Single or Random Spikes)
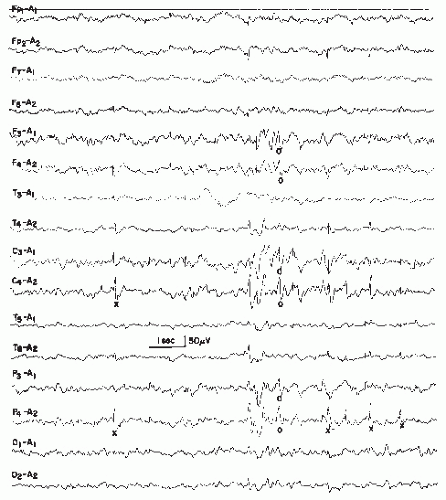
According to IFSECN (6), a spike is a transient, clearly distinguished from the background activity, with pointed peak at conventional paper speed or screen display and a duration from 20 to under 70 msec; the main component is generally negative (Fig. 27.1). Amplitude is variable.
The distinction from the background activity is based on wave morphology and amplitude. In many cases, spikes stand out against the background because of their high voltage. If the voltage of spikes and background activity is approximately equal, the faster character (i.e., the shorter duration) of the spike is its distinctive feature. It is possible that a spike of 50-msec duration and moderate amplitude may be embedded in 20/sec activity (50-msec wave duration), for instance, in a person with epilepsy with considerable drug-induced fast activity. Under these circumstances, the spike activity may be undetectable. In such a case, a fast paper speed or widened screen display could demonstrate the morphologic features of the spike (multiphasic and more pointed character with a dominant negative phase) in contrast with the more monotonous appearance of the fast waves.
Spikes have many characteristics in common and yet there are also remarkable inter- and intraindividual variations and inconsistencies between one spike and the next in the same lead (8). Spikes appear to be hypersynchronous events due to excessive simultaneous neuronal discharge. This view, however, is not congruent with the desynchronized (low-voltage) EEG aspect of “electrodecremental seizures” or the electrode-incremental initiation of focal or generalized attacks. Definitions such as “epilepsy is paroxysmal hypersynchrony” (9) may be correct on the neuronal level but partly incorrect from the viewpoint of the “macro-EEG.”
Basic neurophysiologists have been cautioning against the casual use of terms such as synchronization and desynchronization. Low-voltage fast rhythms are not necessarily desynchronized and may be associated with enhancement and synchronization within intracortical networks (10).
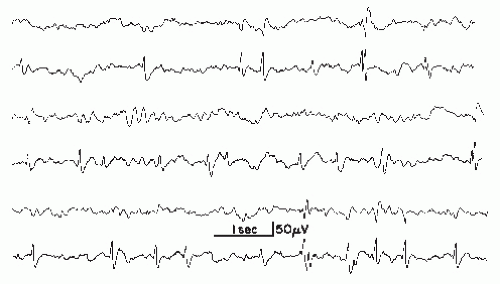 Figure 27.1 Various wave morphologies of spikes recorded from the same patient (age 6 years). Upper lead, T3-T5; lower lead, T5-O1. (First and second tracing from the top, continued in tracings 3 and 4, respectively, 5 and 6.) Note multiphasic character of spikes with predominance of the negative phase (see Chapter 8) and occasional formation of double spikes. |
On the basis of long-term monitoring and computer analysis, Gotman (11) has raised the question of whether interictal spikes are in reality postictal. Gotman (11) and Gotman and Marciani (12) observed marked and prolonged increase of interictal spikes in a patient after a seizure (awake as well as asleep). Drug levels had little influence on the rate of spike activity, and there was no increase of spiking prior to a seizure.
The multiphasic character of a single spike warrants particular emphasis. A sequence of a minor positive, a major negative, and a second minor positive component (Fig. 27.1) is typical in most instances. A slow negative component may trail the spike discharge and often attain about the same amplitude as the negative main component of the spike. This trailing slow component of a single spike should not be regarded as evidence of a spikewave complex. Dutertre (7) in his handbook catalogue shows examples of single spike-wave complexes that one could just as well describe as compounded or multiphasic single spikes. From the vantage point of basic neurophysiology, there is some reason to presume that the trailing slow wave is caused by the same type of hyperpolarization that was demonstrated by Pollen et al. (13) in the experimental analysis of the spike-wave discharge.
The electroencephalographer very seldom finds single spikes on the scalp with predominant positive component (14,15). Positive single spikes are more common in depth recordings; on the scalp, predominant positivity of single spikes raises the question of defective superficial cortical laminae. Following surgery of cortical arteriovenous malformations (AVMs) or after traumatic laceration (16), such positive spikes may be present occasionally. A special type of positive spiking over the vertex has been described by Bergen (17) in a case that combined features of sphingolipidosis and mucopolysaccharidosis. (Also see the work of Engel et al. (18) on the cherry-red spot-myoclonus syndrome.) With dipole formation (especially in benign rolandic epilepsy), a positive spike discharge may be found in a moderate distance from the negative spike focus (19,20).
Spikes represent the basic element of paroxysmal activity in the EEG. A unitarian view that all spikes mean a hidden or overt paroxysmal event would be erroneous. The fine semiology of spikes is extremely important and the EEG interpreter ought to consider the following questions:
What is the precise wave morphology?
Where do the spikes occur?
What is the patient’s age?
What is the state of awareness?
Is there any possibility of an artifact of similar appearance?
Is there any possibility of a physiologic potential of similar appearance?
Discrepancies between spikes and behavioral epileptic events are not uncommon at all. This highly complex situation has been reinvestigated by Niedermeyer (21).
Wave Morphology
The largest and most pronounced spikes are not necessarily associated with more serious epileptic seizure disorders. On the
contrary, rolandic spikes in a child aged 4 to 10 are very prominent; however, the seizure disorder is usually quite benign or there may be no clinical seizures at all.
contrary, rolandic spikes in a child aged 4 to 10 are very prominent; however, the seizure disorder is usually quite benign or there may be no clinical seizures at all.
On the other hand, “small sharp spikes” are indeed marginal spike discharges and will be discussed separately. A very unusual type of rhythmically repetitive and yet interictal spike activity in two epileptic brothers was described by Bauer and Markoff (22).
Spatial Distribution
In childhood, occipital spikes are, in general, the most benign spike discharges, with less than 50% having clinical seizures; rolandic central-midtemporal-parietal spikes are also quite benign, whereas frontal spikes or multifocal spikes are more epileptogenic.
Age
The significance of the age factor is enormous. From the spikes of an epileptic newborn to a seizure focus of old age, age-determined varieties of spikes can be distinguished.
Level of Awareness
Random spikes may occur at any state of waking-sleeping cycle and occur even in rapid-eye-movement (REM) sleep when bilateral-synchronous bursts of spikes or spike waves are usually suppressed.
Relationship to Perceptual Function
Shewmon and Erwin (23,24) investigated the impact of spikes located over the occipital region and its vicinity on visual perception and reaction time in three subjects with posterior spikes. Their work shows that the visuomotor reaction time was prolonged (or the response was absent) when the task was spike-locked. Furthermore, “spike-induced dysfunction was most pronounced when either response hand or visual field of stimulus was contralateral to the spike” (24). In a further study, Shewmon and Erwin (25) emphasized the significance of the trailing slow wave that follows a single spike discharge. The perceptive disturbance was particularly pronounced when the task was presented during the slow wave. These fascinating findings indicate that even single spikes of rapid duration may be capable of momentary impairment of the cortical visual function. This applies to visual tasks. During an ictal-subclinical episode of repetitive fast spiking over the occipital area and its neighborhood, correct mental arithmetic is possible (26).
Distinction from Similar Physiologic Patterns
This differentiation is particularly important in the case of vertex sharp waves during deep drowsiness and stage 2 of light non-REM sleep. In childhood (after age 4), these waves may have a particularly spiky appearance and may be misinterpreted as paroxysmal spikes.
Physiologic sharp or spiky vertex waves are usually quite easily distinguished from rolandic spikes in sleep (Fig. 27.2). Even in the more uncommon case of paroxysmal spike discharges over the vertex, the differentiation from physiologic vertex sharp waves is not difficult.
The physiologic nature of the occipital lambda waves and “lambdoid” activity (positive occipital transients of sleep) must also be considered. The main component of this pattern is positive.
Distinction from Artifacts of Similar Appearance
This distinction depends on the electroencephalographer’s experience and is usually an easy one.
Automatic Spike Detection
Since the pioneering work of Gotman et al. (27) considerable controversy has arisen regarding computer-based automatic detection of spikes in the EEG. The pivotal question is “What should the computer imitate?” (28), and this question hinges on the precise definitions of spikes (29): “A spike detected by any reader is a spike, a spike detected by the majority of readers is a spike, a spike detected by the weighted average of readers is a spike, etc.” According to these authors, average spike attributes are calculated, and the resulting database can serve as a “gold standard” for testing computer algorithms or other readers. With the use of an “off-line” artificial neural network, attempts have been made at real-time detection of spikes (30).
Are Interictal Spikes Nothing but EEG Signals?
Our earlier view (found in this chapter in previous editions)— namely that single spikes are an EEG signal and nothing else— appears to be in need of some correction. This seems to be particularly true for “negative myoclonus” (ictally suppressed motor activity). Beautiful examples have been presented by
Gambardella et al. (31) and Werhahn and Noachtar (32) (also see Ref. 33). A single spike may also cause changes in cerebral blood flow and increase of metabolic demands (34), demonstrated with the simultaneous use of EEG and functional magnetic resonance imaging (fMRI). Even behavioral changes with impaired test performance may be associated with single interictal spikes (35,36). Further information is found in the work of Fisch (33).
Gambardella et al. (31) and Werhahn and Noachtar (32) (also see Ref. 33). A single spike may also cause changes in cerebral blood flow and increase of metabolic demands (34), demonstrated with the simultaneous use of EEG and functional magnetic resonance imaging (fMRI). Even behavioral changes with impaired test performance may be associated with single interictal spikes (35,36). Further information is found in the work of Fisch (33).
Sharp Waves
According to IFSECN (6), a sharp wave is a transient, clearly distinguished from background activity, with pointed peak at conventional paper speed or screen display and duration of 70 to 200 msec, that is, more than approximately 1/14 to 1/5 seconds. The main component is generally negative (Fig. 27.3).
Jasper (37) pointed out that the rising phase of the sharp wave is of the same order of magnitude as in spikes, but the descending phase is prolonged. The configuration with a steeper ascending phase, however, is not always present.
A very unusual type of repetitive slow sharp wave activity has been reported in prematurely born infants with intraventricular hemorrhage; these discharges show predominantly positive polarity and are recorded mainly over the rolandic region (38,39). The maximum of this activity is sometimes found over the vertex.
Spikes and sharp waves are neurophysiologically closely related phenomena; both of them are typical paroxysmal discharges and highly suggestive of an epileptic seizure disorder, although both phenomena may occur in patients without a history of seizure disorder.
Sharp waves are usually found as random focal discharges; most anterior temporal spikes are, in a strict sense, sharp waves. This is also true for most benign rolandic spikes of childhood. Sharp waves are more seldom found in generalized-synchronous bursts where single spikes, spike waves, and polyspikes predominate.
It has been contemplated that sharp waves on the scalp correspond with spikes in the depth or on the cortex. Combined depth and scalp recording clearly refutes this view (40). One can detect spikes as well as sharp waves in depth recordings; a deep sharp wave usually corresponds with a sharp wave on the scalp if there is any corresponding scalp activity at all.
The long duration of a sharp wave permits better insight into the multiphasic character of this potential. A small preceding positive component may be very fast and qualify as a spike; even a small biphasic spike discharge may precede the much larger sharp wave. Some sharp waves even exceed the maximum length of 200 msec of the IFSECN definition (6) (Fig. 27.4), also named “slow sharp waves” (41) or, in cases of particularly long duration, “blunted sharp waves” (Fig. 27.4). Others become very complex. They consist of a constantly varying number of components; such compounded sharp waves may occur as the periodic discharges (periodic lateralized epileptiform discharges [PLEDs]) of Chatrian et al. (42).
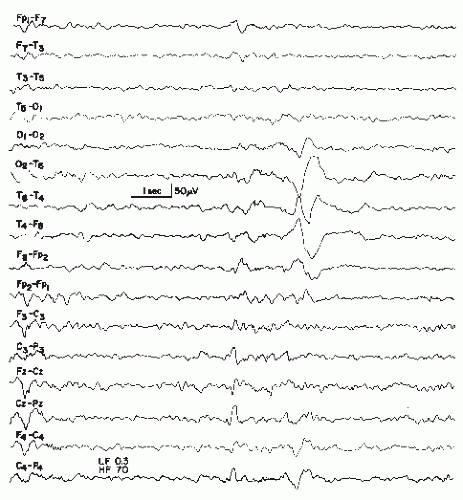 Figure 27.4 An example of a very slow sharp wave (blunted sharp wave), maximal over T6, obtained from a 6-year-old boy with craniocerebral trauma at age 4 followed by seizures. |
It is certainly not incorrect to use the terms spikes and sharp waves synonymously when a local paroxysmal event is discussed, although purists of nomenclature would regard this as a breach of etiquette.
Polyspikes or Multiple Spikes
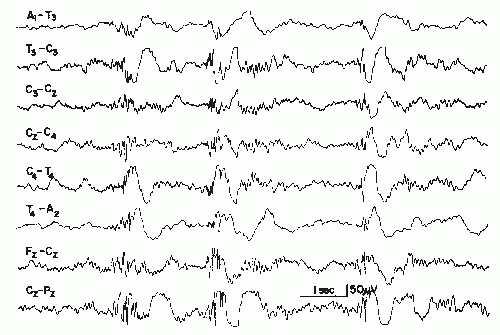
This discharge type represents a complex of spikes and may also be called polyspike complex. In modern terminology (6), the term multiple spike complex is preferred on the grounds of linguistic considerations, because “polyspikes” is an etymological hybrid. It has been defined as a complex paroxysmal EEG pattern with close association of two or more diphasic spikes occurring more or less rhythmically in bursts of variable duration, generally with large amplitudes (Figs. 27.5 and 27.6) (7).
Polyspike bursts are readily elicited by electrical stimulation of single depth leads, especially in limbic regions. On the scalp, however, polyspikes occur mostly as bilateral- or generalized-synchronous discharges. Exceptional focal polyspikes are occasionally encountered; these usually have a frontal maximum, except for occipital accentuation in hypsarrhythmia. Polyspikes and also polyspike-wave complexes are sometimes associated with concomitant myoclonus, especially in primary generalized epilepsy and in photosensitive individuals with this type of seizure disorder. Children with Lennox-Gastaut syndrome may also show association of polyspikes and myoclonus.
Runs of Rapid Spikes
This pattern has been described as “grand mal discharge” (43,44), “fast paroxysmal rhythms” (45), “rhythmic spikes” (46), and “generalized paroxysmal fast activity” (47). It is seen only in sleep and occurs in older children, adolescents, and younger adults (Fig. 27.7). It consists of bursts of spike discharges at a rate from 10 to 25/sec, usually generalized but with a well-defined maximum over frontal regions; it may even be confined to the frontal leads. The voltage is in the medium to high range, often exceeding 100 or even 200 µV. The discharge rate is in most cases somewhat irregular. The bursts last for about 2 to 10 seconds; bursts of more than 5-second duration are usually associated with tonic seizures and thus represent an ictal pattern.
The obvious misnomer “grand mal discharges” is based on certain similarities with the ictal EEG of a generalized tonic-clonic seizure. In a patient population with such seizures, runs of rapid spikes as an interictal pattern are a very rare finding. This has been confirmed by the data of Chayasirisobhon et al. (48). The inappropriateness of the term grand mal discharge was stressed by Rodin et al. (49), who thought that this pattern occurred in patients with primary generalized epilepsy who suffered from more than one type of seizure and especially from akinetic seizures. As already noted by Gastaut et al. (46), this discharge is very typical in patients with Lennox-Gastaut syndrome; it is hardly ever found outside this clinical entity (50, 51 and 52). It may be found in very rare instances of posttraumatic epilepsy with EEG traits of the Lennox-Gastaut syndrome (53). Brenner and Atkinson (47), however, insist that runs of rapid spikes (“generalized paroxysmal fast activity”) may be found outside the Lennox-Gastaut syndrome. There is evidence that runs of rapid spikes may occur in all “imitators” of the Lennox-Gastaut syndrome (54).
We know little about the neurophysiologic underpinnings of this pattern. It is clearly a frontal lobe discharge, and the associated tonic seizures are probably arising from the premotor and supplementary motor portions of the frontal lobes. There could be certain similarities to the decremental seizure patterns, which also originate most commonly from the frontal lobe (1).
Unfortunately, those electroencephalographers who never use sleep portions in routine records will never see this fascinating pattern, which is definitely in need of further basic research.
Three-Per-Second Spike-Wave Complexes
Classical 3/sec spike-wave complexes are widely known even outside the community of electroencephalographers. The discharge is officially termed spike- and slow-wave complex (6); this term comprises all types of spike-wave complexes, which are listed separately because of markedly differing associated clinical-epileptologic conditions. The official definition (6) is quite simple: “A pattern consisting of a spike followed by a slow wave.” Older terms such as spike-and-dome complex, wave-and-spike complex, and dart-and-dome complex are seldom used these days and should be discouraged. The abridged term 3/sec spike wave is certainly acceptable, because the omitted word complex is automatically implied.
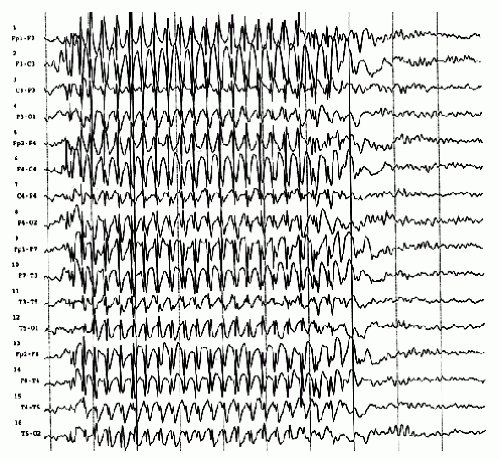
Spike-wave complexes of the classical variety (3/sec) have been described as the EEG pattern of petit mal absences (Fig. 27.8) (44).
Since that time, the 3/sec spike-wave discharge has been equated with absence epilepsy. Regrettable mistakes have occurred due to lack of communication between electroencephalographers and clinicians who initiated treatment with anticonvulsants specific for absence seizures due to the use of the term 3/sec spike-wave complexes alone. The term petit mal discharge (43) has reinforced this type of reflex thinking. Fortunately, electroencephalographers and epileptologists have always cautioned against the misinterpretation of the term petit mal discharges; this should be interpreted as “discharges that occur in petit mal absences but also in other conditions.” The works of Silverman (55), Clark and Knott (56), and Lundervold et al. (57) have clearly shown that the spike-wave discharge as an interictal event correlates with the occurrence of absence seizures only in a moderate percentage of patients (16% according to Clark and Knott).
Since that time, the 3/sec spike-wave discharge has been equated with absence epilepsy. Regrettable mistakes have occurred due to lack of communication between electroencephalographers and clinicians who initiated treatment with anticonvulsants specific for absence seizures due to the use of the term 3/sec spike-wave complexes alone. The term petit mal discharge (43) has reinforced this type of reflex thinking. Fortunately, electroencephalographers and epileptologists have always cautioned against the misinterpretation of the term petit mal discharges; this should be interpreted as “discharges that occur in petit mal absences but also in other conditions.” The works of Silverman (55), Clark and Knott (56), and Lundervold et al. (57) have clearly shown that the spike-wave discharge as an interictal event correlates with the occurrence of absence seizures only in a moderate percentage of patients (16% according to Clark and Knott).
The 3/sec spike-wave discharge is a worthwhile subject for more detailed discussion. As to wave morphology, there is good evidence that the spike-wave complex is not simply an association of a spike and a slow wave, although the above-mentioned definition is justifiable as a simplification. The spike-wave complex contains hidden components that can be seen even with simple visual analysis at 30 mm/sec paper speed or equivalent screen display; special recording methods will reveal these extra components much better. Cohn (58) and Weir (59) have elucidated the components of this pattern. Most important is a spike of positive polarity during the descending portion of the slow wave (59), becoming evident as notching under usual recording conditions.
On the basis of experimental studies of cortical faradization, Jung and Toennies (60) stressed the inhibitory character of the slow wave following a spike discharge (“Bremswelle”). Experimentally induced spike-wave-like activity in cats, elicited by electrical stimulation of thalamic intralaminar structures, was studied with intracellular cortical microelectrodes (13,61,62). According to Pollen (61), all components of the spike-wave complex were the “consequence of postsynaptic activity.” This author points out that “the initial positivity which may precede a negative spike (depending upon the intensity of the stimulation) results from depolarization of middle and deep lying neurons, whereas the surface negative spike is the result of EPSP [excitatory postsynaptic potential] generation primarily upon apical dendrites of vertically oriented pyramidal type neurons.” According to Pollen (61), the long surface negative wave is generated by inhibitory postsynaptic potentials (IPSPs) predominantly on or near the soma of similar pyramidal cells. Thus, the slow wave indeed appears to be related to inhibitory impulses.
From this vantage point, the spike-wave discharge apparently represents an alternating succession of excitation and inhibition. The clinical-ictal activities are thus constantly curbed by intervening inhibitory impulses that prevent the attack from progressing into massive downward discharges with motor effects (polyspikes with massive myoclonus) or into a full-blown tonic-clonic attack. For this reason, motor manifestations of ictal episodes characterized by spike waves are almost always inconspicuous or modest. Furthermore, spike-wave bursts rarely proceed into generalized convulsions; such rare exceptions have been demonstrated by Halász (63) and Niedermeyer (64).
The distinction between classical (3/sec), slow (2 to 2.5/sec), and fast (4/sec) spike-wave complexes and the smaller 6/sec spike-wave discharge is justified on the basis of different clinical-epileptologic correlates of each spike-wave type; it is most definitely not an example of electroencephalographic hair splitting. The classical 3/sec spike-wave discharge is most typical and most pronounced in children with absence seizures. Clinical absences are usually present when the burst lasts for longer than 5 seconds. Thus, shorter bursts are usually subclinical, but numerous psychophysiological attempts have been made to demonstrate certain fluctuations of level of awareness even in apparently subclinical spike-wave bursts. The classical spike-wave complex is, in most cases, easily activated by hyperventilation, whereas the slow and the fast forms of this discharge show little or no enhancement.
A classical 3/sec spike-wave burst does not run exactly at a rate of 3/sec. The complexes are faster at the onset of the burst (mostly around 4/sec), and then slow down to 3.5 and 3/sec for the main portion, and eventually slow to 2.5/sec at the end of the burst. During a burst, the spike discharges become gradually smaller (Fig. 27.8), often shrinking to insignificance as in drowsiness and sleep.
The spatial distribution of the bursts is very typical; the maximum almost always lies over the frontal midline region, whereas the minimum is found over temporal and occipital areas. Because somewhat different findings obtained by Dondey (65) were based on EEG recordings in which no midline electrodes were used, they do not invalidate the concept of a frontal midline maximum, which has been confirmed by Rodin and Ancheta (66) with the use of a brain mapping technique. The understanding of this distribution type is highly conducive to a better comprehension of the underlying mechanisms. Almost all spike-wave bursts are bilateral synchronous or generalized
synchronous; the synchrony, however, is not perfect (58,67). The age of distribution lies mainly in the range from 4 to 16 years.
synchronous; the synchrony, however, is not perfect (58,67). The age of distribution lies mainly in the range from 4 to 16 years.
The work of Lemieux and Blume (68) has shed more light on the spatial distribution of the spike-wave complex on the basis of computer analysis. According to these authors, field distribution of spikes differed from that of the ensuing slow waves. Spikes “arose in one frontal region and propagated to the homologous part of the other frontal region with a peak-to-peak interval less than 15 msec. Less commonly, spikes first moved anteriorly within the initiating frontal region before contralateral propagation occurred.” By contrast, negative slow waves were more diffuse, more symmetrical in evolution, and more posteriorly centered than the spikes.
The onset of the spike component in one frontal lobe is congruent with the earlier findings of Cohn (58) and Lüders et al. (69), who noted asynchronies ranging from 5 to 20 msec. The frontal lobe is a large structure and subdivisions are necessary. Onset of spike-wave activity (and, in particular, of the spike component) over the frontal midline means in reality that a frontal portion in the immediate vicinity of the interhemispheric fissure is the origin of the discharge; this area belongs to the supplementary motor zone.
As to the neuropsychological correlates of subclinical generalized spike-wave bursts and clinical absences with spike waves, it should be pointed out that the impact of apparently subclinical 3/sec spike-wave bursts might be stronger than one would expect from routine clinical observation. With its maximum over the frontal midline (frontal lobe close to interhemispheric fissure), there might be some impact on prefrontal lobe functions and, in particular, on working memory. The concept of working memory, introduced by Baddeley (70) and enormously refined by Fuster (71,72), implies a system of constant perceptory afferences to the prefrontal dorsolateral region whence motor impulses emanate, resulting in readiness (set) for motor action. This system could be blocked for a moment by a few spike-wave complexes (or even by a single one) and interfere with working memory. In a clinical 3/sec spike-wave absence, blocking of working memory might be the most likely explanation for the mild disturbance of consciousness and its immediate full recovery with the termination of the attack (73). No “true disturbance of consciousness”—epileptic or nonepileptic—can recover immediately. In other words, the function of working memory is simply suspended for the duration of the spike-wave complexes.
Due to the uniqueness of primary generalized epilepsy with 3/sec spike-wave complexes in the human (74), animal models for this response have great limitations. The arrest reaction of the monkey (75) produced by alumina cream injection into the fronto-orbital cortex has some resemblance to the human absence epilepsy. However, this cannot be said about the sudden freeze of motility noted in the cat stimulation of the cat’s mesial anterior thalamus (76), since there is no spike-wave activity associated with this type of arrest reaction.
According to Sperling and Skolnick (77), there is a general decrease of cerebral blood flow (by 133 xenon method) during human generalized spike-wave activity, which, however, is less pronounced in frontal regions than in the parietal lobes. Transcranial magnetic stimulation of the motor cortex in
patients with primary generalized epilepsy and 3/sec spikewave complexes produced motor evoked potentials of significantly reduced size when the cortical stimulus was time-locked to the slow-wave component of the spike-wave complex (but slightly reduced or unchanged when the stimulus was time-locked with the spike) (78). This underscores the special inhibitory character of the slow-wave component, which has been known since the work of Jung and Toennies (60).
Slow Spike-Wave Complexes
After the demonstration of the 3/sec spike-wave complex and its relationship to the petit mal absence (44), the Gibbses and William G. Lennox were struck by the occurrence of slow spikewave complexes in a much different type of patients with seizures other than absence. The classical 3/sec spike-wave complex was termed petit mal discharge. Consequently, the slow spike-wave complex was termed petit mal variant discharge. This term was used first by Gibbs et al. (79). In that study, hypoglycemia was induced in order to demonstrate an increase of classical 3/sec spike-wave activity in the hypoglycemic state; an increase of slow spike-wave activity, however, was not elicited with this activation method when used in appropriate patients.
The wave morphology of this pattern varies considerably. In the majority of the cases, the complex consists of a rather slow spike (according to the definition, a sharp wave, lasting 70 msec or longer) and a slow wave. In a sizable number of cases, true spikes (60 msec or less duration) are followed by a slow wave (Fig. 27.9).
The spatial distribution is, in most instances, quite similar to that of the 3/sec spike-wave complex. In the vast majority of the cases, the bursts are bilateral or generalized synchronous (with imperfect synchrony); a frontal midline maximum is the rule in these discharges. Lateralization or occasional focal occurrence is sometimes observed, usually in children with severe residual brain damage in certain areas where parenchymatous destruction abolishes the spike-wave discharge.
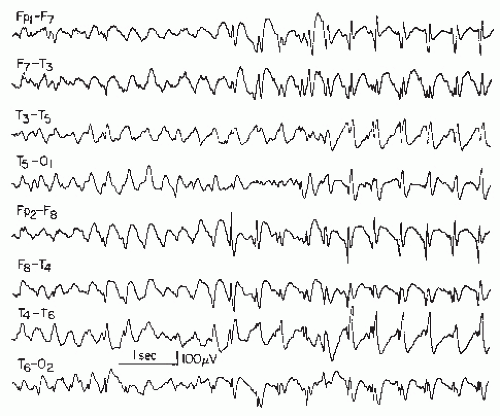 Figure 27.9 Generalized slow spike-wave complexes (mostly around 2/sec) in a child with severe epileptic seizure disorder (Lennox-Gastaut syndrome). |
Generalized slow spike-wave discharges are often quite prolonged. In some children or adolescents, the entire sleep portion (light and moderately deep non-REM sleep) consists of unabated generalized slow spike-wave activity. The diagnosis of an “electrical status epilepticus” may or may not be justified in such cases; there are usually no behavioral or polygraphic characteristics to suggest an ongoing ictal event. In some cases, the slow spike-wave activity may be found only in non-REM sleep.
Although the classical 3/sec spike-wave complex is seldom seen before the age of 4 and almost never before 3.5 years, its slow counterpart appears much earlier, sometimes before the age of 6 months. At this early age, the frontal maximum may not be readily demonstrable.
The slow spike-wave complex is almost always associated with a severe and often uncontrollable type of childhood epilepsy (seldom with onset between ages 11 and 20 years) called Lennox-Gastaut syndrome (46,50,52,79,80). Most of these children show a variety of minor attacks and evidence of mental retardation.
Thus, the distinction between slow (1 to 2.5/sec) and classical (3/sec) spike waves is of great clinical significance. One has to keep in mind, however, that many children or adolescents with slow spike waves also show series of 3/sec or even 4/sec spikewave complexes; the presence of these spike-wave types is obviously of no significance in such cases and what counts is the slow type. On the other hand, patients with classical 3/sec spike-wave complexes and a usually benign type of epileptic seizure disorder almost never show slow spike waves except when a 3 or 4/sec spike-wave burst may slow down to 2.5/sec at the end.
Fast Spike-Wave Complexes
This pattern is closely related to the classical 3/sec spike-wave complex; both discharge types are most commonly lumped together. A differentiation, however, is justified on clinical grounds.
Gastaut (81) deserves credit for an outline of the distinctive features between the 3 and the 4/sec (or 4 to 5/sec) spike-wave discharge. According to his investigations, the fast spike-wave burst is usually of shorter duration (1 to 3 seconds), it occurs in patients older than 15 years, the bursts are always subclinical, and the associated seizure disorder is usually characterized by myoclonic jerking, grand mal attacks, or a combination of both seizure types, whereas petit mal absences are quite uncommon. Paroxysmal flicker responses are common in such patients. A positive family history of epileptic seizures disorder is frequently found in this group of patients. The 4/sec spike-wave discharge is spatially distributed in the same manner as the 3/sec spike-wave discharge; the frontal midline maximum is quite prominent.
Six-Per-Second Spike-Wave Complexes
This is a marginal paroxysmal pattern, described by Gibbs and Gibbs (43) as follows: “The spike in this pattern has a strong positive component but the entire wave complex looks in general like a miniature reproduction of the 3 per second spike and wave of petit mal.” In view of the positive component of the spike, Silverman (82) has stressed similarities to the 6/sec component of the 14 and 6/sec positive spike discharge; he also
observed transition from the former to the latter with deepening drowsiness. Marshall (83) introduced the term wave and spike phantom, which has found only limited acceptance.
The clinical correlates of 6/sec spike-wave complex have been elucidated by Thomas (84), Gibbs and Gibbs (85), Hughes et al. (86), and Thomas and Klass (87). It is usually a pattern of adulthood but may also occur in adolescents and children. About 50% to 60% of the patients suffer from clear epileptic seizures (mostly generalized tonic-clonic); the remainder show a history of syncopal attacks, posttraumatic states, or (as emphasized by Small (87)) psychiatric problems.
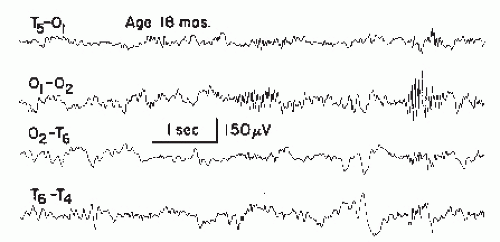
The 6/sec spike-wave complex is an uncommon but not rare pattern (about 0.5% to 1% in a central EEG laboratory). The discharge may be recorded in waking state, drowsiness, and light non-REM sleep; light drowsiness appears to be the optimal recording condition (Fig. 27.10).
Investigations regarding the spatial distribution of this pattern have led to an important dichotomy. Hecker et al. (88) pointed out that a distinction must be made between frontal accentuation and occipital accentuation. We are evidently dealing with two different types of discharge; the frontal type is most commonly associated with epileptic seizure disorders and sometimes found in combination with other paroxysmal discharge types. The occipital types are found predominantly in patients with no evidence of epileptic seizure disorder.
In a study of 839 patients with 6/sec spike-wave complexes, Hughes (89) placed particular emphasis on the distinction between frontal and occipital maximum. He summarized the
principal features of both forms by the acronyms WHAM and FOLD:
principal features of both forms by the acronyms WHAM and FOLD:
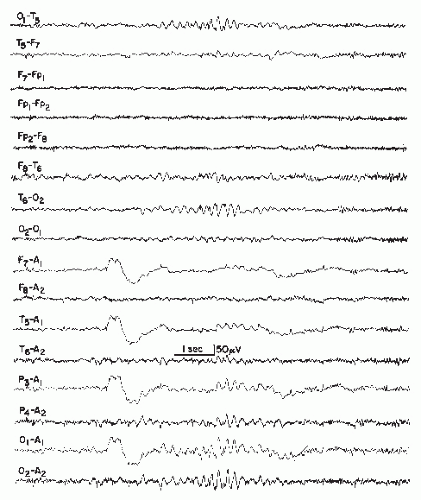 Figure 27.10 A short run of 6/sec spike waves, posterior type, recorded in a 52-year-old woman with a history of head injury 2 years earlier and subsequent headache, dizziness, and memory loss. There was computed tomography (CT) scan evidence of cortical atrophy.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|