Introduction
Epileptic seizures and other kinds of epileptic activity are emergent phenomena from the interactions between cells organized into networks. To understand the relationship between the dynamics of cellular and local networks activity and epileptic discharges requires simplified models: This chapter outlines the use of in vitro preparations and computer models in this task. The chapter starts by describing different types of in vitro preparations and models of epilepsy, before outlining general aspects of computer modelling, and finally describing pathophysiologic mechanisms involved in epileptic phenomena.
In Vitro Preparations
Invertebrate preparations in vitro played an important role in the development of cellular studies of epilepsy, but they have largely been superseded by mammalian preparations, which are more directly relevant to the problem of epilepsy. In vitro preparations share the advantages of (a) mechanical stability, which is very helpful for cellular electrophysiology in which microelectrodes must to be precisely positioned in, on, or near neurons; (b) direct visualization, using suitable microscopes, of either general anatomic features or individual cells, both of which may be used for optical recording of Ca2+, membrane potential, and other physiologic variables; and (c) simpler control over extracellular ions and drugs due to the absence of a blood–brain barrier. The following paragraphs outline the main preparations of mammalian brain tissue in vitro, starting from the simplest and going on to consider the brain slice in more detail.
Dissociated cell. Neurons can be used for studies of their intrinsic properties shortly after they have been dissociated from tissue by mechanical agitation and enzymatic digestion of the extracellular matrix. This is a powerful technique for studying channelopathies in chronically epileptic tissue. Dendrites are amputated, which improves space clamp, but, of course, loses the contribution of all but the most proximal dendrites to the neuron’s properties.42,145
Dissociated culture. Neurons dissociated from pieces of brain tissue can be plated into culture dishes and maintained for several weeks. They will rewire into synaptic networks that can sustain epileptic activity. The limiting case is the single neuron culture that makes extensive connections with itself and generates spontaneous epileptic discharges.106 Dissociated cultures are useful for those methods that take some time to work and/or need good access to neurons, such as transfection with molecular constructs to modify neuronal behavior. Non-neuronal cultured cells play a crucial role in identifying phenotypes associated with mutations found in monogenic epilepsies (see the section on chronic epilepsies). Cultured cells may also be used in the high-throughput screening of drugs with well-defined targets such as sodium channels.23
Brain slice. This probably is the most widely used in vitro preparation in basic epilepsy research, and is the main topic of this chapter.38 It is a section of brain tissue, a few hundred microns thick, maintained in physiologic saline or artificial CSF solution that contains the salts, glucose, and oxygen needed to sustain life. The thickness is a compromise between the need to retain the neuronal circuitry and the need to keep the centre of the slice alive, most importantly by maintaining a high enough partial pressure of oxygen. In practice, brain slices preserve enough neuronal circuitry to sustain at least some kinds of epileptic activity. The underlying principles of brain slice preparation date back to the pioneering biochemical work of Warburg, during the 1930s. Typically, brain slices will survive several hours, perhaps a day or two.
Organotypic slice culture. Slices prepared under aseptic conditions can be maintained for several weeks in appropriate tissue culture media. These slice cultures tend to flatten onto the substrate on which they are grown, which can be advantageous for visualization and manipulation.49 They lend themselves to gene transfection and similar mid- to long-term manipulations that are much more difficult to achieve in simple slices.12,41 One disadvantage is that the local connectivity changes as amputated axons die and surviving axons sprout, although this can be comparable to changes observed in chronic experimental and human epilepsies. Slice cultures allow the study of processes underlying damage and subsequent reorganization over prolonged periods.76 Slice cultures lend themselves to high-throughput screening of potential anticonvulsants with a more realistic end point than the single ion channel or receptor used for similar work on isolated cells.121
Isolated brain region. Seizures normally involve larger areas of tissue than can be preserved in a typical slice. To overcome this limitation, it is possible to cut slices in directions that preserve some longer-range pathways, for example, between the entorhinal cortex and hippocampus,6,148 between the two hippocampi,71 or the thalamus and cortex.32
Isolated brain. The guinea pig brain can be isolated and perfused, providing many of the benefits of an in vitro preparation combined with in vivo connectivity.78 Its main advantage is the maintenance of long range-connec- tions.51 Its main disadvantage is that it is difficult (but not impossible) to maintain the tissue in good condition.
Table 1 Cellular Actions of Acute Convulsant Treatments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The choice of the most appropriate preparation depends on the question being asked. Brain slices from experimental animals have proved particularly useful for most issues addressed in this chapter, both the acute actions of convulsants and the pathophysiology of chronic models of epilepsy. It is possible to apply in vitro techniques to human tissue removed during surgery for medically intractable seizures. This method has obvious limitations on the number and consistency of samples, and controls require some ingenuity—and usually comparison with chronic animal models.31,48,74,154 Slices from human epileptic foci usually do not produce spontaneous epileptic activity, but can generate some kinds of synchronous activity,74 and can reveal stronger than normal responses to convulsant treatments such as increased potassium (K+)48 and 4-aminopyridine5 (Table 1).
Perhaps the most commonly used brain slices in epilepsy research are from the hippocampus, and this chapter draws extensively from that literature. Similar principles, however, apply to other epileptogenic parts of the brain.
In Vitro Models of Epilepsy
In vitro models can be divided in two main groups: (a) acute convulsant treatments of normal brain tissue and (b) in vitro (or “ex vivo”) studies of chronically epileptic tissue.64 Acute models really model symptomatic seizures rather than epilepsy, but they have yielded major insights that can be applied to the abnormal brain tissue responsible for chronic epilepsy in vivo. Hypersynchronous epileptiform activity can be induced by perfusing normal brain slices with a variety of convulsant compounds (see Table 1).65,67,127,128,132,137 Many of these agents block synaptic inhibition mediated by γ-aminobutyric acid (GABA)A receptors, but others can work by increasing neuronal excitability, strengthening excitatory synaptic receptors, or altering synaptic release. The challenge was to find out how acute changes in synaptic or other neuronal properties can make the normal circuitry of the brain generate epileptic activity.
The prolonged exposure of hippocampal-entorhinal cortex slices to either low magnesium (Mg2+) or 4-aminopyridine22,39 results in a state characterized by recurrent epileptic discharges that are resistant to most current antiepileptic drugs. They therefore provide in vitro models of drug-resistant seizures, an area for which novel antiepileptic drugs are most urgently needed.
Chronic models are also amenable to study in vitro, simply by making the preparation from a chronically epileptic laboratory animal (or perhaps from human patients undergoing surgical resection to treat medically intractable epilepsy). The more common chronic models of epilepsy112 can be divided into (a) those that start with status epilepticus and are associated with substantial cell loss30 (including those using systemic or intracerebral kainic acid,13,98 systemic pilocarpine,140 and sustained electrical stimulation50,84,86); (b) those not associated with status or with early lesions (including kindling87 and intracerebral tetanus toxin62,66); and (c) genetic models (most commonly of absence seizures94,114). Essentially all these models alter neuronal structure and function in ways that reduce seizure threshold, often to the extent that seizures occur spontaneously. A central challenge for work on these models in vitro is to determine, at the subcellular, cellular, and network levels, how the properties of neurons, synapses, and circuits have changed in chronically epileptic tissue.
Introduction to Computer Modelling of Neurons
Epileptic discharges represent an emergent property of neuronal networks. Understanding the functional relationships between the properties of individual neurons and of the networks they form is difficult on the basis of experimental observations alone. Computer modelling can play a crucial role in linking these different levels of analysis.
Computer Simulations of Single Neurons
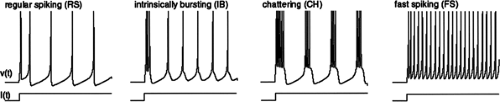
Simulations of single neurons come in a variety of flavors. The level of abstraction depends on the questions asked. Neurons can be highly simplified to “integrate and fire” formulations. Inputs to these model neurons produce a shift in their potential, which will decay with some time constant, forming essentially a simple circuit of a capacitance and resistor; a threshold detector determines whether each cell will generate an output. Such neurons have a much more limited repertoire of firing patterns than do real cortical neurons, in which the majority are regular spiking cells, a large minority are intrinsically bursting neurons (and many of the regular firing cells can be changed to intrinsically bursting by changing the ionic environment8), fast spiking cells (all are interneurons), and chattering cells (in the neocortex but not the hippocampus24). The underlying physiology is described in more detail in Chapter 29. It is possible to create model neurons using relatively simple equations that capture these firing patterns (Fig. 1).60 Such equations are based on neuronal physiology but do not attempt to simulate individual ion conductances. They are computationally efficient and are particularly useful for modelling large scale networks.99a
An alternative approach, which is the basis of most of the modelling work described here, uses equations to represent specific ion channels known to be present in particular kinds of neuron. Such realistic neuronal simulations build on the pioneering work of Hodgkin and Huxley,57 who showed that action potentials can be described quantitatively as ion conductances that are functions of membrane potential and time, with the time-dependence usually including components for activation and inactivation. The representation of specific classes of ion channel by differential equations allows a rather direct comparison of computer model and biologic experiment by using drugs and toxins that have well-defined effects on specific ion channels. Most importantly, this allows predictions made by such realistic models to be tested, and falsified, experimentally.
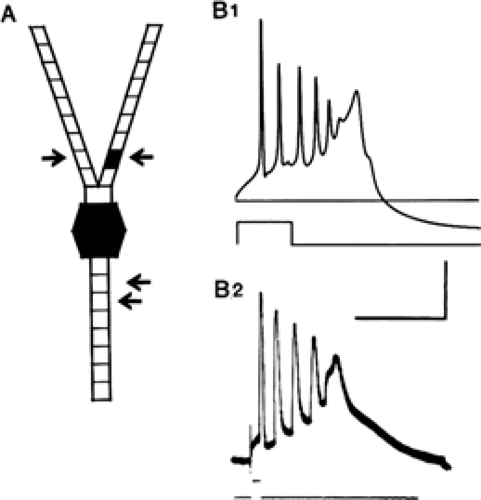
Mammalian neurons are anatomically and physiologically much more complex than is the squid giant axon. Rall et al.97 developed the approach of collapsing the complex anatomy of the dendrites and somata of neurons to electrically equivalent cylinders that are more amenable to computation than is the real anatomy. Traub et al.133 developed compartmental models of hippocampal pyramidal neurons (Fig. 2), which have been refined extensively131,134,137,138 and extended to many other types of neuron. Models of this kind include several key features:
Passive electrical properties (membrane resistance and capacitance and intracellular resistance), usually of a series of linked “compartments.” These are notional isopotential cylindrical segments that represent parts of the neuron, and together produce time and length constants similar to those in real neurons. The appropriate number of compartments depends on the purpose. Even those that use dozens generate new insights into dendritic function.131 More detailed models of branched dendrites have revealed the complex processing of synaptic inputs.73,115 Epileptic activity usually affects branches uniformly so that simpler nonbranched models often suffice.
Ion channels. These are represented by equations of their dependence on voltage and time and, where appropriate, intracellular calcium (Ca2+) or extracellular neurotransmitters. Individual currents, and the ion channels that generate them, are relatively well characterized. (Individual channels switch between two, or in some cases several, states; at the level of the whole neuron, however, their collective effects are more easily represented by Hodgkin-Huxley–type equations.) One complication that often arises occurs when the experimental evidence on specific currents is collected at subphysiologic temperatures; this data must be adjusted to the near-physiologic temperatures usually needed to sustain epileptic activity.
Distribution of ion channels throughout the neuron, particularly on its dendrites and soma. These data are difficult to collect, but a reasonable body of work, largely from patch clamp, has been developed.68,88 The distinct distributions of ion channels in different classes of neuron is an argument for using multiple rather than single compartments in models. A complication here is that dendritic ion channels are plastic and change in response to physiologic and pathophysiologic factors, including epilepsy.46
The quantitative details of ion channels usually will vary between individual real neurons,83 and often will not be known precisely, so models should be robust to reasonable variation in their parameters; models that need very precise tuning to reproduce biologic reality need particularly critical evaluation. Once a model has been developed, it must be tested against real experimental data to ensure that its response to various kinds of stimulation and pharmacologic experiment match reality—or, perhaps more importantly, to identify where it fails so that the model can be refined or revised. Toxins and drugs exist that block or modulate most channels, and they can and should be used to test falsifiable predictions made by the model.131 In the case of the hippocampal CA3 pyramidal cell, the intrinsic bursting depends on voltage-gated calcium channels in the dendrites, initially located in one dendritic compartment of the Traub mode 1 (see Fig. 2), but now distributed throughout the dendrites, in line with more recent electrophysiologic evidence.88
Just because a model reproduces the properties of a neuron does not mean it simulates how the real neuron works. In a revealing study, Prinz et al.95 sought an alternative to “hand-tuning” model neurons. They tested a large (∼1.7 × 106) database of a single-compartment model of the lobster stomatogastric neuron, in which the maxima for eight membrane conductances were varied systematically. They showed that multiple distinct combinations of ion channels could generate specific physiologic responses, and that the effects of varying a particular ion conductance could depend on the values of the other conductances in the cell. Interestingly, some neurons can adapt to changes in the expression of one ion channel, such
as IA(an outward potassium current), by changing the expression of another, such as Ih(hyperpolarization-activated inward current).80 Similar simulation database approaches are feasible, if more difficult, in multicompartment models. These simulation studies suggest that much more physiologic evidence is needed on the diversity of intrinsic and synaptic properties (and on their combinations within individual neurons) in both normal and epileptic tissue.
as IA(an outward potassium current), by changing the expression of another, such as Ih(hyperpolarization-activated inward current).80 Similar simulation database approaches are feasible, if more difficult, in multicompartment models. These simulation studies suggest that much more physiologic evidence is needed on the diversity of intrinsic and synaptic properties (and on their combinations within individual neurons) in both normal and epileptic tissue.
Simulation packages such as Neuron and Genesis make the building of compartmental models considerably easier than once was the case. Databases of typical models of many types of neurons are available, for example NeuronDB.91
Computer Simulations of Neuronal Networks
Building all but the simplest of synaptic networks remains less straightforward than making compartmental models of single neurons. Several projects exist that attempt to make relatively user-friendly packages for simulating networks, but they are not readily accessible to the nonspecialist. Several generic issues must be considered whatever the technical approach. Some form of randomization of neuronal properties across the population of model neurons is essential to avoid spurious synchronization. Real neurons will vary in their properties, both functional and structural, but experimental evidence on the extent of this variation is limited and often dismissed as experimental noise. That such variation exists is increasingly clear.25,35,46,99,101
Synaptic connectivity can be difficult to quantify experimentally in a statistically realistic way. Pairs of neurons will have some probability of a direct connection, but that probability will depend on distance and direction, among other variables. If these data are available, it should be fairly straightforward to generate a similar pattern of connections. A simple exponential decay of the density of excitatory synapses with distance from the parent soma reproduced many features of the propagation of epileptic activity through the hippocampus.130 More precise information on the geometric organization of synaptic connectivity will help constrain network models.110 The number of connections that must be modelled grows rapidly with population size, analogous with the Internet where, for example, Metcalfe’s law argues that total connectivity scales as the square of the number of members of the network.
Simulations of small networks show that disparate combinations of synaptic and intrinsic properties can result in similar network dynamics,96 and indeed, that the real networks in different animals may adopt different strategies to achieve the required output.25 Despite such difficulties, network simulations have provided real insights into epileptic activity, as shown by the work outlined here. However, the potential for different sets of network mechanisms to result in similar emergent properties96 emphasizes the importance of these network models to make experimentally testable, falsifiable predictions. The experimental tools are drugs and toxins, possibly applied focally, and molecular modifications (with the caveat that endogenous functional compensation can occur80,122).
Real neuronal networks have more complicated properties than do the models outlined so far. For example, synapses may facilitate or depress with repetitive use, with the direction of the change depending on the synapse and (critically) on the temperature.72 Synapses may exhibit long-lasting potentiation (LTP)17,36(see Chapter 35), and they may experience pre- and postsynaptic modulation.33,82,113 Extrasynaptic receptors exist that, on the whole, have not been incorporated into compartmental models, but have significant impact on excitability.77,107
Lumped Computer Models
Compartmental models link directly with experimental neurophysiology and pharmacology in vitro, and they play a critical role in the work outlined within this chapter. Other approaches, however, can provide valuable insights. The use of simpler models of spiking neurons was mentioned earlier.60,99 Other models use a “lumped” approach; in these models, the properties of individual neurons within a population are lumped together into equations that relate the population’s net inputs to its net outputs. The lumped equations include elements for specific classes of synapse on the main types of neuron involved, and normally include a transfer function to relate the mean membrane potential of a population of neurons to their overall firing rate. This style of model has a long history (approaching five decades), back to the seminal work of WJ Freeman and others. Lumped models can, and should, make experimentally testable predictions. They have proved useful, particularly in the context of the thalamocortical system, where they have addressed issues such as the bistability of the electroencephalogram (EEG) between interictal and ictal states, and the potential therapeutic use of counterstimulation to reduce the frequency and/or duration of seizures.120,151 Although these particular studies addressed absence seizures, mainly in vivo, such methods have also been used in vitro, for example in the hippocampal low-Mg2+ model, to study the dynamics of seizure-like events120 or to model stereoencephalographic signal recorded from depth electrodes.150
Computer models have contributed greatly to the understanding of the underlying mechanisms of epileptic activity, particularly of the acute actions of convulsants on normal brain tissue.
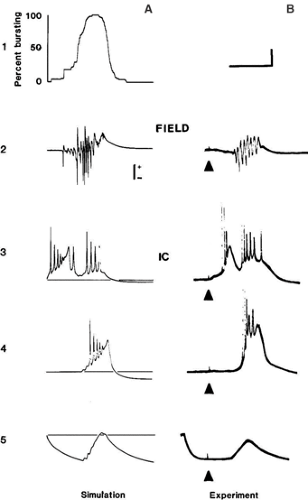 FIGURE 3. Simulation and experimental recordings of single synchronized bursts evoked by a localized stimulus in the disinhibited guinea pig hippocampal slice. The epileptic field potential (line 2) occurs after long latency in both simulation (A) and experiment (B). The simulation is of a network of the model neurons of FIGURE 2, sparsely connected by realistic excitatory synapses. Most cells generate PDSs synchronously with this field potential (line 4), and injecting hyperpolarizing current into the recorded cell reveals the underlying giant EPSP (line 5). A rare cell (line 3) fires a burst soon after the stimulus, along with another burst during the field event. The long latency corresponds to generations of exponential growth of the event; this takes time, because burst propagation can last tens of milliseconds. From Traub RD, Wong RKS. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–747, with permission. |
Epileptic Neurons and Mechanism of Interictal Spikes
Many acute convulsants result in brief synchronous discharges that look like interictal spikesb on the EEG or electrocorticogram (ECoG) from epileptic foci in humans. Some of the earliest intracellular studies in vivo (on penicillin foci) showed that these discharges were associated with substantial depolarizations and rapid action potential firing in most of the neurons in the epileptic focus. These abnormally large depolarizations were called paroxysmal depolarization shifts (PDSs) (Fig. 3B).84
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree