CHAPTER 70 Epilepsy Surgery
Outcome and Complications
The past 25 years has witnessed the evolution of epilepsy monitoring and surgery into a mature neuroscience subspecialty.1,2 The proliferation of multidisciplinary epilepsy centers has increased our capacity to provide multidisciplinary evaluations of patients with intractable epilepsy, thereby leading to greater numbers of patients being offered surgical treatment (Table 70-1). A robust clinical literature has documented both improvements in surgical approaches and a set of measures of surgical outcomes that has stimulated changes in our approach to these patients. In addition, the emergence of a nosologic context of “surgically remediable syndromes” has been spurred by advances in brain imaging and studies of the neurobiologic underpinnings of epileptogenesis.
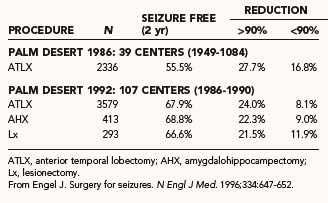
TABLE 70-1 Worldwide Surgical Procedures for Temporal Lobe Epilepsy
| PROCEDURE | BEFORE 1986* | 1986-1990† |
|---|---|---|
| ATLX | 2336 | 4862 |
| SAH | — | 568 |
| Neocortical resection | 825 | 1073 |
| Lesionectomy | — | 440 |
ATLX, anterior temporal lobectomy; SAH, selective amygdalohippocampectomy.
From Engel J. Surgery for seizures. N Engl J Med. 1996;334:647-652.
Traditional en bloc resective approaches, which predominated during the first 60 years of epilepsy surgery, have been supplemented by more focused, minimalistic approaches, including selective amygdalohippocampectomy (SAH), multiple subpial transection (MST), keyhole deafferentation hemispherotomy, radiosurgery, and neuromodulatory approaches such as deep brain stimulation (DBS), vagal nerve stimulation (VNS), and the NeuroPace device (Table 70-2).
TABLE 70-2 Surgical Approaches for Epilepsy
Advances in our understanding of the intractable epilepsies have facilitated a discrete taxonomy of “surgically remediable syndromes” (Table 70-3).1 Each syndrome and each new treatment approach engender a new data set with regard to (1) seizure, neurobehavioral, and psychosocial outcomes; (2) complications (surgical, neurological, neuropsychological, and psychobehavioral); (3) “health outcomes” expressed in terms of alterations in quality of life (QOL); and (4) “cost-effectiveness” in comparison to alternative therapeutic interventions.
TABLE 70-3 Surgically Remediable Syndromes
| Temporal lobe epilepsy (TLE) | Idiopathic Mesial temporal sclerosis/mesial temporal lobe epilepsy Lesional (tumor, vascular malformation, developmental, ischemic, traumatic) |
| Extratemporal epilepsy | Idiopathic Lesional (tumor, vascular malformation, developmental, ischemic, traumatic) |
| Catastrophic epilepsy | Lesional Hemimegalencephaly Diffuse cortical dysplasias Sturge-Weber syndrome Rasmussen’s encephalitis Porencephalic cysts |
| Secondarily generalized epilepsies | Lennox-Gastaut syndrome |
Epidemiology and Health Care Costs of Intractable Epilepsy
Epilepsy is a relatively common disorder, with more than 2 million Americans (1.3%) afflicted and approximately 1 in 10 Americans having a minimum of one seizure over the course of a lifetime.3 Up to a third of all individuals with epilepsy are refractory to medical therapy.4 Medically intractable epilepsy is costly. Estimates of the lifetime cost of intractable epilepsy incorporate both direct cost (cost of medical care) and indirect cost (i.e., productivity, lost earnings). In a 2000 study, patients with medically intractable epilepsy in the United States were found to account for 42% of the estimated $1.7 billion in annual direct medical costs for epilepsy and 86% of the $10.8 billion in indirect costs.5 Beyond these estimates, the burden of epilepsy incorporates additional, unmeasurable indirect costs that encompass pain, suffering, and reduction in the QOL of epileptic patients and their caretakers.6,7
Advances in Outcome Assessment of Epilepsy Surgery
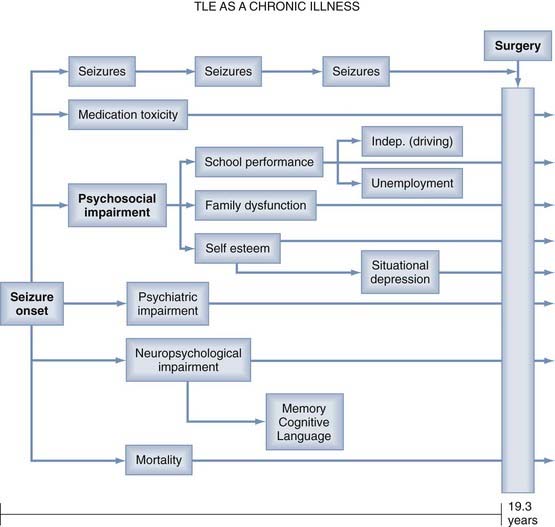
Seizure frequency, previously considered the “gold standard” of outcome measures, is an inadequate measure of surgical outcome for many reasons. Persistent epileptic seizures are often associated with significant psychosocial, psychiatric, and neuropsychological impairments, as well as medication toxicity and excess mortality rates (Fig. 70-1).8 These disabilities may persist despite relief of seizures after “successful” surgery. Therefore, contemporary studies of postoperative outcome emphasize neuropsychological and psychosocial functions and “health outcomes” commensurate with the World Health Organization’s definition of health as “… a state of complete physical, mental and social well-being …”.3,8
Classification of Seizure Outcomes
Studies have demonstrated that “health-related quality of life” (HRQOL) and psychosocial measures may not improve significantly with as much as a 70% reduction in seizure frequency after surgery.9 In recognition of the benefits of postoperative freedom or near freedom from seizures, contemporary seizure outcome classification schemes have emphasized patterns of seizure reduction that are likely to have an impact on QOL.10–12 The Engel classification scheme (Table 70-4) provides four categories of seizure outcome: I, seizure free; II, “rare” seizures (two to three per year): III, “worthwhile improvement” (>90% reduction); and IV, “no worthwhile improvement” (<90% reduction).8,11 That a class IV (“no worthwhile improvement”) outcome may be associated with a 70% reduction in frequency is emphasized by HRQOL studies showing that epilepsy-specific measures are affected even by a few seizures per year.
TABLE 70-4 Engel’s Outcome Classification
From Engel J. Surgery for seizures. N Engl J Med. 1996;334:647-652.
Neuropsychological Outcomes
Neuropsychological assessment is useful both during the surgical patient selection process and as a tool to assess outcomes after surgery. The aim of the evaluation is to establish a profile of the patient’s strengths and weaknesses in multiple domains on a variety of standardized tests and questionnaires (Table 70-5) in relation to normative values derived from the general population.13
TABLE 70-5 Neuropsychological Assessment of Epileptic Patients
| FUNCTIONS | TESTS |
|---|---|
| Sensory functions | Halstead-Reitan examination |
| Motor functions: dexterity, coordination, speed, flexibility | Purdue Pegboard, Grooved Pegboard, Thurstone’s Uni- and Bimanual Coordination Test |
| Perceptual-motor functions | Beery Visuo-Motor Integration Test, Block Design, Rey-Osterrieth Complex Figure |
| Psychomotor development and intelligence | Griffith or Bayley Developmental Scales, Wechsler Intelligence Scales for Adults or Children (WISC, WAIS), Stanford-Binet |
| Attention | Concentration Endurance Test, Auditory Continuous Performance Test |
| Memory and Learning | |
| General | Wechsler Memory Scales for Children and Adults |
| Verbal: word lists, story recall | California Verbal Learning Test (CVLT) |
| Visual: faces, patterns | Rey-Osterrieth Complex Figure |
| Expressive: sentence construction | Boston Naming Test, Token Test |
| Receptive: comprehension | Peabody Picture Vocabulary Test (PPVT) |
| Written: reading, spelling | Wide Range Individual Achievement Test (WIAT) |
| Numerical operations | WIAT, Woodcock-Johnson Achievement Battery |
| Executive functions | Tower of London, Wisconsin Card Sorting Test, Fluency Tests |
| Personality | Rorschach, Thematic Apperception Test for Children or Adults, SCL-90R |
| Affective state | Beck Depression Inventory, Hamilton Anxiety Scale |
| Social adjustment | Vineland Adaptive Behavior Scales, Achenbach Child Behavior Check List (CBCL) |
| Quality of life | Quality of Life in Epilepsy questionnaires (e.g., QOLIE-31, QOLIE-AD-48) |
Of particular concern in temporal lobe surgery are losses of memory function, including the rare but disabling syndrome of global amnesia, as well as the more common material-specific memory losses affecting short-term verbal memory in the language-dominant hemisphere and visual-spatial memory in non–dominant-hemisphere, temporal lobe operations. The Wada test, which was originally developed to determine hemispheric lateralization of language function,14 was subsequently adapted by Milner and colleagues15 to provide a measure of the risk for loss of memory function postoperatively. The Wada test has been used for many years to identify patients at risk for global memory loss,16 and in fact, such losses are uncommon since the Wada test was universally adopted. However, reports of favorable memory outcomes in patients who failed the Wada test preoperatively (“false positives”) have called into question the reliability of this procedure in some patients.17 The Wada test has also been useful in identifying lateralized temporal lobe dysfunction, which may correlate with the side of seizure onset,18,19 the likelihood of a favorable seizure outcome, and more recently, prediction of the risk for material-specific memory loss (particularly verbal memory loss) after surgery.20,21 Nonetheless, the Wada test is invasive and requires a degree of patient cooperation, which may be suboptimal in young children and mentally retarded patients. Wada test results may be difficult to interpret in patients with bilateral language representation, excessive agitation, insufficient hemispheric inactivation by amobarbital (Amytal), or other procedural factors.13,22 Functional neuroimaging modalities (i.e., functional magnetic resonance imaging [fMRI], [18F]fluorodeoxyglucose positron emission tomography [FDG-PET], and [99mTc]-hexamethylpropyleneamine oxime single-photon emission computed tomography [HMPAO-SPECT]) are increasingly being used as noninvasive tools for localization of epileptogenic cortex and assessment of focal functional deficits in patients with intractable epilepsy.23–26 [18F]FDG-PET, by imaging the rate of cerebral glucose metabolism, reflects neuronal losses and focal functional deficits in epileptic patients and serves as a predictor of postoperative seizure outcomes. In 70% to 90% of patients with temporal lobe epilepsy (TLE), interictal [18F]FDG-PET detects unilateral temporal hypometabolism or asymmetric bitemporal hypometabolism. This reflects neuronal loss in the damaged temporal lobe, correlates with clinical and neurophysiologic findings,24–26 and has been associated with favorable postsurgical seizure control in several studies.25,27,28 Patients with left mesial temporal lobe epilepsy (MTLE) and regional left hemispheric hypometabolism tend to have impairments in verbal memory and word fluency.29 Although the laterality of hypometabolism as determined by [18F]FDG-PET is related to memory deficits measured by the Wada test,30–33 the location or spatial pattern of the metabolic changes that can predict impairment in Wada memory performance has not been characterized. If future studies can demonstrate a strong correlation between Wada memory scores and metabolism on [18F]FDG-PET, presurgical [18F]FDG-PET could play a role in predicting memory laterality during the presurgical evaluation.
fMRI represents neuronal activity indirectly via hemodynamic changes in the brain. The size of the cortical area activated and the number of involved neurons directly influence the magnitude of the changes in regional cerebral blood flow.34 Studies comparing fMRI and Wada test results in the same patient have shown a high correlation (from 80% to 100%) between the two procedures when the studies were aimed at either investigating language lateralization35–38 or assessing memory asymmetries.39–41
Health-Related Quality of Life
Over the past decade, epilepsy-specific HRQOL instruments have been developed to assess the impact of epilepsy surgery on the “health” and “quality of life” of patients afflicted with intractable epilepsy. These instruments incorporate “generic” measures of health status previously validated in studies of health outcomes in other disease states, as well as “epilepsy-specific” measures of health status, which are more sensitive to the cognitive, memory, and role limitation issues implicit in the disease of intractable epilepsy.8,42,43 Contemporary outcome studies incorporate “health outcomes” measures in which the individual patient’s perspective becomes an integral aspect of the health care outcomes assessment.44 To facilitate HRQOL studies in epilepsy surgery patients, assessment tools such as the Epilepsy Surgery Inventory (ESI-55) have been developed and validated for use in this population.42 This instrument incorporates a “generic core” adapted from the RAND 36-Item Health Survey, along with 12 epilepsy-specific items addressing the domains of cognitive function, role limitations because of memory problems, and health perceptions.45 Other instruments developed to assess HRQOL have included the Quality of Life in Epilepsy—89 (QOLIE-89)46 and a shorter version, the QOLIE-10.44
Cost-Effectiveness
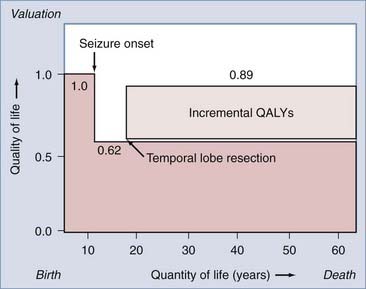
The reduction in the morbidity associated with chronic epilepsy achieved with epilepsy monitoring and surgery appears to be accompanied by parallel reductions in long-term health care costs and unemployment. Recent advances in epidemiologic and “health outcomes” investigations have facilitated cost-effectiveness studies of epilepsy surgery. Cost-effectiveness studies incorporate established patient preferences for being in certain states of health, along with cost estimates of each health state, to assess the cost-effectiveness of surgical intervention. “Health states” are typically quantified on a scale of 0 to 1. For example, preference-based values for epilepsy-related health states include a healthy, disease-free patient (1.0), a patient with intractable complex partial seizures (0.62), and a postoperative, seizure-free patient (0.89).47 This approach permits calculation of “quality-adjusted life years” (QALYs) by multiplying the expected postoperative survival in years by the appropriate quality adjustment factor (from 0 to 1), which reflects the outcome state.47,48 The number of QALYs added to a patient’s life by successful epilepsy surgery can be estimated (Fig. 70-2) and, when combined with the cost of therapy, can be expressed as the cost, in dollars, of providing an incremental QALY to the life of a patient with intractable epilepsy. The “cost-effectiveness” of surgical intervention can then be expressed in various ways to provide a measure of the cost-effectiveness of epilepsy surgery that can be compared with other unrelated health care interventions.
Complications of Diagnostic/Surgical Procedures
Epilepsy surgery is safe and effective. Nevertheless, invasive diagnostic procedures and definitive surgical interventions do carry some risk, which must be considered when recommending surgical intervention to patients with intractable seizures.8
Intracarotid Amytal Procedure (Wada Test)
Complications of the Wada test encompass the complications of transfemoral carotid angiography, including thromboembolism and stroke (0.5% to 1.0%), allergic reactions to contrast agents (1 in 40,000), and local complications of femoral artery puncture.49 Rare mortality has been reported.16
Depth Electrodes
For many years, depth electrodes were used routinely as part of the surgical evaluation of patients with intractable epilepsy.50 In contemporary practice, noninvasive assessments, including structural MRI scans and improved surface electroencephalographic (EEG) monitoring techniques, have reduced the requirement for invasive recordings. An advantage of depth electrodes is that low-voltage, localized discharges emanating from medial structures, including the amygdala and hippocampus, may be detected as evidence of the site of seizure onset. Depth electrodes are invasive and associated with risk for infection (1% to 4%) and intracerebral hemorrhage, which has been noted to occur in 3% of parasagittal placements and 1% of lateral placements.49 Rare mortality from depth electrodes has been reported to be caused by lacerations of large parasagittal draining and bridging veins or the posterior cerebral artery, as noted in one older series of 163 patients.51 The use of modern stereotactic techniques has reduced the morbidity associated with depth electrodes, and recent studies report few complications with no permanent neurological deficits in most cases.52,53 One study has suggested that a postoperative decline in verbal memory may occur with the implantation of hippocampal depth electrodes bilaterally.54
Subdural Strip Electrodes
Subdural strip electrodes have provided a safe alternative to brain penetration by depth electrodes.55 Strip electrodes are usually placed symmetrically over suspected sites of seizure onset and yield excellent recordings from neocortical structures. Although recordings from intracerebral sites are not provided with this modality, these electrodes do not require cortical penetration with its associated risks. The principal risk with subdural electrodes is infection, which may be manifested as superficial infection, meningitis, or brain abscess, as reviewed in an extensive series of 350 patients.55 Other reported adverse events have included fever with temperatures higher than 102°F, migraine, and temporalis muscle fibrosis, as indicated in a prospective study of 55 patients. In another recent multicenter study, only five minor complications occurred in 131 patients, three of which were reported to be small hematomas not requiring evacuation.56
Subdural Grid Electrodes
Subdural grid electrodes provide electrographic tracings from a large expanse of cortex and permit extraoperative brain mapping to localize eloquent functions in relation to the epileptogenic zone. Grid electrode placement requires a large craniotomy and the egress of numerous electrode cables through the scalp for the duration of the monitoring period (usually 1 to 2 weeks). The grid is removed at a second craniotomy, during which the definitive cortical resection is performed. It is not surprising that bone flap infection and meningitis are prominent concerns as complications of this procedure. Infection rates of 22% were identified in an early Cleveland Clinic series but declined to 7% when cables were tunneled to exit percutaneously.57 A contemporary series of 49 patients undergoing grid implantation reported a 4% infection rate, subdural hematoma formation requiring emergency evacuation in 8%, and brain swelling in 2%.58 Other series have reported subdural hematoma formation in 8% and increased intracranial pressure and brain shift requiring premature removal of the grid.49 Meticulous surgical technique with avoidance of injury to or compression of large cortical or bridging veins, tunneling of electrode cables, administration of perioperative antibiotics, and full utilization of mannitol and dexamethasone (Decadron) are likely to improve outcomes and prevent complications.49
Resective Surgery
Temporal Lobe Epilepsy
Intractable epilepsy of temporal lobe origin is the most common syndrome for surgical consideration. It is thus no surprise that the majority of outcome data in the literature have focused on temporal lobe surgery. The syndrome of mesial temporal lobe epilepsy typically incorporates a history of an early insult in infancy or childhood,59,60 hippocampal sclerosis and atrophy on MRI,61 an abnormal creatine–N-acetylaspartate (NAA) ratio on magnetic resonance spectroscopy (MRS),62 temporal hypometabolism on interictal PET,63,64 and a characteristic pattern of hyperperfusion and hypoperfusion on ictal SPECT.59 EEG studies reveal an anteromedial epileptogenic zone, and Wada testing reveals appropriate memory deficits.8,65 Histopathologic analysis of resected hippocampi reveals loss of principal hippocampal neurons, synaptic reorganization, sprouting of mossy fibers, and enhanced expression of glutamate receptors.8,66,67
A smaller population of patients with “cryptogenic” TLE have normal MRI findings preoperatively.60 Patients with “lesional” TLE have temporal lobe neoplasms, vascular malformations, disorders of cortical development, or traumatic/ischemic insults within the temporal lobe. These lesions may variably involve mesial temporal lobe structures or may be associated with hippocampal sclerosis (“dual pathology”)68 and thus lead to distinct surgical approaches and outcomes.
Mesial Temporal Lobe Epilepsy
Pathologic Substrate
The pathologic features of mesial temporal sclerosis (MTS) include (1) loss of principal neurons (atrophy), (2) glial proliferation (gliosis), and (3) sprouting of dentate granule cells.69,70 When the loss of hippocampal principal neurons exceeds 50%, hippocampal atrophy is visible on MRI69 and gliosis will be manifested as high signal on fluid-attenuated inversion recovery (FLAIR) and T2-weighted images. Not uncommonly, some degree of hippocampal sclerosis is present contralateral to the side of resection and may not be detected by standard MRI assessment. Bilateral MTS is visible on MRI scans less commonly (5% to 10%).71
Seizure Outcomes
Imaging, Neuropsychological, Electrographic, and Clinical Features
A variety of preoperative factors have been recognized over the years as identifiers of more favorable postsurgical outcomes with regard to seizure control. Although seizures were well controlled in 91% of patients with MRI-defined unilateral MTS, the favorable outcome response to surgical intervention declined sharply to 62% in patients found to have bilateral MTS and was notably worse (only 50%) in patients without MTS.72 However, depth electrode recordings in patients with radiographic bilateral MTS have suggested that some may have only a unilateral onset, and in this subset of patients excellent seizure outcomes may still be achieved, as evidenced in a small study of 5 such patients, 4 of whom were completely seizure free after 2 years of follow-up.71 It has also been found in several studies that hippocampal hypometabolism noted on PET studies is strongly correlated with MRI findings of MTS and histologically confirmed neuronal atrophy and gliosis in this region,73,74 thus outlining the prospective utility of PET in preoperative evaluation. In a similar vein, another study evaluating postsurgical patients seen at 1-year follow-up reported an 83% seizure-free rate in those with preoperative unilateral hippocampal hypometabolism,64 in sharp contrast to just a 38% seizure-free rate in patients with either normal PET studies or evidence of multilobar hypometabolism. In a 5-year outcome assessment of 135 operated patients with imaging evidence of lesions, MTS, and normal hippocampi, 80% of patients with lesions, 62% of patients with MTS, and 36% of patients with normal hippocampi were found to be seizure free 2 years postoperatively.75 Multivariate analysis may improve the prediction of outcome.76 MTS, a “known cause” of epilepsy, and the absence of generalized seizures portend a satisfactory outcome. Satisfactory outcomes were achieved in 78% to 83% when both of these features were present, in 53% to 61% when one was present, and in 29% of patients when neither was present.77 Preoperative evaluation with the intracarotid Amytal test (Wada test) has suggested that lateralizing memory deficits are independently predictive of a seizure-free outcome.20,65
Lateralization of seizure onset can be identified with either interictal78,79 or ictal76,80,81 EEG monitoring. In patients with nonlesional TLE, favorable seizure control was noted most often in those with concordance between their interictal EEG and MRI findings.82 A 5-year follow-up study of 28 patients demonstrated that in 26 of them, a 75% reduction in seizures was noted in those who had a unilateral anterior to middle temporal epileptiform focus without discordant findings in other studies, the majority of whom (61%) were reported to be seizure free.79
In a recent review of 126 articles on temporal lobe resection published between 1991 and 2001, the median seizure-free rate was 70%, and of 63 factors analyzed, favorable outcomes were associated with (1) preoperative MTS, (2) anterior temporal localization of interictal epileptiform activity, (3) absence of preoperative generalized seizures, and (4) absence of seizures in the first postoperative week.83 Age at seizure onset, preoperative seizure frequency, and extent of lateral resection had no association with outcome.
Age at the time of surgery (i.e., >45 years old) may be related to seizure outcome, with some studies suggesting less favorable outcomes84 and others suggesting that older age does not predispose to a less favorable outcome.85,86
Surgical Versus Best Medical Management
A 5-year follow-up study compared the seizure outcomes of 148 operated and 94 nonoperated patients with TLE.87 Freedom from seizures during the final year of follow-up was achieved in 62% of operated and 7.5% of nonoperated patients. Complete seizure freedom over the entire study period was achieved in 44.6% of operated and 4.3% of nonoperated patients. None of the nonoperated and 8.8% of the operated patients were free of the need for antiepileptic drugs (AEDs) at follow-up. Other adult studies have documented AED freedom in 21% and 35% of patients at 1- and 2-year follow-up, respectively.88,89 Pediatric studies have documented 30% to 44% AED-free rates at 2 years78,89 and 30%, 35%, and 60% AED-free rates at 2, 5, and 10 years postoperatively, respectively.90 The only prospective, randomized, controlled study of surgical versus best medical management for TLE compared the outcomes in 40 medically managed and 40 surgically managed patients.2 At 1-year follow-up, 58% of the surgically treated and only 8% of the medically treated patients were free of seizures impairing consciousness.
In general, systematic reviews suggest that 66% to 70% of patients are seizure free at short- term (<5 years) follow-up.83,91–94 Long-term (>5 years) follow-up studies show that 41% to 79% of patients remain seizure free after temporal lobe resection87,91,93,95–99 and that 15% to 20% of patients have relapses after initial seizure freedom at 5 to 10 years after surgery.92,93,96,100
Surgical Approaches to Mesial Temporal Lobe Epilepsy
The traditional “en bloc temporal lobectomy” incorporated a 5- to 6-cm lateral resection along with a portion of the amygdala and anterior hippocampus.8,101,102 More centers now use a focused anteromedial resection in which restricted resection of the middle and inferior temporal gyrus is combined with thorough hippocampal removal.8,103–105 Transsylvian SAH permits exclusive resection of medial structures.8,106,107 Awake surgery with intraoperative electrocorticography (ECoG) and functional brain mapping permits tailored resection of both lateral and medial structures.8,108,109
Extent of Cortical and Hippocampal Resection
In the early era of epilepsy surgery, lateral resection alone often yielded disappointing results with regard to freedom from seizures.110 More recent studies in which lateral and mesial structures were resected have suggested that the extent of lateral resection does not correlate with seizure outcome.111,112 One study reported that 53 of 100 patients were seizure free after a standard lateral resection was combined with complete amygdalectomy and minimal hippocampal resection.113 The favorable outcomes after SAH,11,114 the effectiveness of removal of any residual posterior hippocampus in reoperative surgery,115–117 and the identification of posterior hippocampal onsets in depth electrode studies103 all suggest that thorough hippocampal resection may be essential to optimize seizure outcomes.8 Although earlier reports suggested that the extent of mesial resection had no association with seizure outcome, two more recent studies addressed this issue effectively and came to different conclusions.83 In the first study, postoperative MRI was used to confirm the extent of mesial resection in 94 TLE patients and revealed a correlation of the extent of mesiobasal resection with seizure outcome, regardless of the extent of lateral resection.117 In a separate, prospective randomized controlled trial, 70 patients with unilateral ictal onsets confirmed on intracranial recordings underwent temporal lobe resection and were randomized to partial or total hippocampectomy.105 At 1- and 2-year follow-up, the group that underwent complete hippocampectomy experienced superior seizure outcomes (69% versus 38% seizure free at 1 year) without increased neuropsychological or neurological morbidity.
Impact of Surgical Approach
Although a small percentage of TLE patients may harbor epileptogenic zones exclusively in the lateral temporal neocortex,118 the majority of candidates for nonlesional temporal lobe resection have the syndrome of MTLE, for which resection of mesial structures is emphasized.8,59 A long-term follow-up study of 50 patients managed with lateral resection alone revealed 44% to be seizure free at follow-up.119 In Engel’s compilation of seizure outcomes from 107 centers worldwide, outcomes were similar between centers regardless of the surgical approach, provided that the mesial structures were adequately resected.1 In single-center studies, SAH and standard resections produced similar results.72 In Yasargil’s SAH series, 22 of 30 (73%) patients with TLE and depth electrode–confirmed hippocampal onsets were seizure free postoperatively.114 These results after SAH compare favorably with studies of patients with histologically confirmed MTS undergoing standard anteromesial resections.120
A single-center study reported on the seizure outcomes of 321 patients who underwent various temporal lobe resections between 1989 and 1997.121 This series incorporated 96 standard anterior temporal resections, 84 restricted lateral and generous mesial resections, and 91 SAH procedures. The notable finding was the absence of significant differences in seizure outcome between the three operative cohorts in which different resection strategies were used.8
Improvement in Seizure Outcomes Over Time
Early investigations of temporal lobe resections documented seizure-free outcomes in 27% to 44% of patients in long-term follow-up.122–125 This was during an era in which lateral resection was emphasized. Engel compared worldwide outcomes of earlier (1949 to 1984) and more recent (1986 to 1990) eras and documented superior outcomes in contemporary series (Table 70-6).1,8,10 This was thought to result from improved methods of patient selection and convergence of surgical resection approaches to emphasize medial resection. Ultimately, in many patients seizure control is not static and may fluctuate over time.
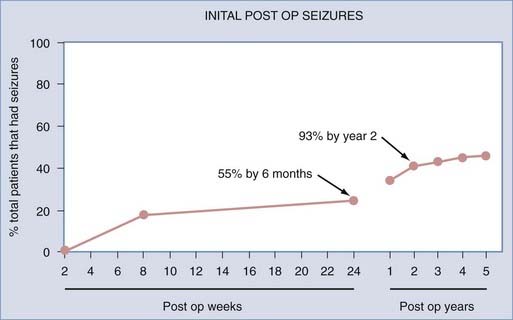
In a study of patients with refractory TLE by Sperling and colleagues, 89 patients who underwent anterior temporal lobe resections between 1986 and 1990 were monitored postoperatively for 5 years.12 Approximately 70% of the patients reported being seizure free over the past year (Engel class I). Only 55% of the patients were seizure free over the entire 5-year period after surgery. In those in whom seizures did develop, 55% experienced them within the first 6 months postoperatively, and almost 93%, relapsed within the first 2 years after surgery (Fig. 70-3). This suggested that in patients who were seizure free for the first 2 years after surgery, recurrent seizures were unlikely to develop thereafter.
A similar outcome study reviewed 148 patients who had an overall 44.6% seizure-free rate after 5 years, 62% of whom were seizure free in the previous year.87 Another 5-year outcome study demonstrated similar results, with an overall 50% seizure-free rate at 5 years and a similar 62% seizure-free rate in the last 2 years of the study.75
Acute Postoperative Seizures and Late Improvement
Traditionally, immediate postoperative seizures were considered incidental to the trauma of surgery itself and not predictive of surgical outcome, analogous to early seizures after stroke or traumatic brain injury, which have not been shown to be reflective of long-term seizure outcomes.10 This “running down” phenomenon was initially characterized by Rasmussen, whose early studies suggested that it occurred in as many as 15% to 20% of patients.126–128 Recent investigations have not confirmed this belief, with only 5% of patients with TLE and immediate postoperative seizures experiencing improved seizure control over time. “Running down” may occur more commonly in patients with unilateral epileptiform discharges preoperatively.129 Other studies, however, suggest that immediate postoperative seizure activity may predict poorer long-term seizure control in both adults130,131 and children.132
Intraoperative Electrocorticography
For many years, temporal lobe resections “tailored” on the basis of intraoperative ECoG provided a standard surgical approach in many centers. Recent experience suggests that standardized anatomic resections may produce similar outcomes, and the appropriate role of intraoperative ECoG is not well defined in current practice. Early studies suggested that intraoperative ECoG may be useful to predict outcome, particularly if epileptiform discharges are present or absent in either preresection or postresection cortex.128,133,134 Ojemann’s recent study used ECoG to define the extent of hippocampal resection in an attempt to both optimize seizure outcomes and minimize postoperative memory deficits though hippocampal sparing.109 In contrast, two studies in which standardized anatomic resections were performed in the context of intraoperative ECoG before and after resection failed to identify any predictive value with regard to ultimate seizure outcomes.135,136 Other studies suggest a limited value of ECoG as a guide to the extent of resection for TLE.111,137,138 Another study in patients with lesional temporal or extratemporal epilepsy in which resection was carried to normal tissue margins found that the extramarginal spike distribution was not associated with seizure outcome.139 In contemporary practice, in which patient selection is guided by advanced imaging and video-EEG data, intraoperative ECoG is not used in some centers in the context of defined surgically remediable syndromes.83
Neuropsychological Outcomes
Cognitive Outcome
Intellectual function is generally preserved in adults12 and children140 after temporal lobe resection, and when seizure control is achieved, improvement in some measures has been reported. In the Graduate Hospital series of 89 consecutive patients undergoing dominant- and non–dominant-hemisphere resections, measures of verbal IQ were unchanged postoperatively and the study demonstrated improvements in performance and full-scale IQ.12 In part, these improvements were believed to be attributable to practice effect. Subsequent studies have reported similar findings, including improvement in verbal IQ after non–dominant-hemisphere resections and performance IQ after dominant-hemisphere resections.141–144
Global Memory Deficits
Although uncommon in modern practice, global amnesia is a disabling complication of temporal lobe surgery. Two patients with global amnesia were described in an early Montreal Neurological Institute series of 90 dominant-hemisphere temporal resections.145 These patients exhibited a syndrome of profound anterograde memory loss with preservation of cognitive performance, personality, early memory, and technical skills.146,147 Earlier reports had described global amnesia after unilateral resection in either the dominant or nondominant hemispheres.143,147,148 Evidence that hippocampal rather than lateral neocortical removal is critical to the production of global amnesia is provided by a report of a patient undergoing a staged resection in whom global amnesia occurred only after the hippocampus was removed.145 This is further supported by reports of global amnesia after SAH.149 Contemporary series report rare postoperative global memory deficits at a frequency of less than 1%,49,143,150,151 whereas a less profound postoperative “severe amnesia” may be more common.152
Material-Specific Memory Deficits
Reported “material-specific” memory deficits include loss of short-term verbal and nonverbal memory postoperatively. In particular, short-term verbal memory loss is common after dominant temporal lobe resections, with significant decrements in verbal memory being reported in 25% to 50% of operated patients.153 Verbal memory loss may accompany resections in the nondominant hemisphere, although at a much lower frequency.153 Nonverbal memory deficits are less commonly identified, even after non–dominant-hemisphere resections,154 although some authors report that these losses may be obscured by “practice effects.”153 In the Graduate Hospital series, evidence of significant short-term verbal memory loss was identified in many patients after dominant-hemisphere temporal lobe resections, with a trend toward improvement after nondominant temporal lobe resections.12 In a recent 10-year series of 321 TLE patients undergoing a variety of surgical approaches for the treatment of nonlesional and lesional TLE, verbal memory declined in 34%, improved in 19%, and remained stable in 46% of patients.121 Weak preoperative performance on measures of verbal memory, young age at surgery, and operations on the nondominant side were associated with stability or improvement in verbal memory. Short-term nonverbal memory measures exhibited similar rates of improvement and deterioration. Weak preoperative performance on measures of nonverbal memory and dominant-side operations were associated with improvement, whereas advanced performance preoperatively and older age were associated with deterioration.121
The high frequency at which verbal memory impairment occurs after dominant-hemisphere temporal lobe surgery has stimulated interest in predicting which patients are at risk for postoperative deficits.8 Recent studies have documented significantly greater risk for verbal memory loss in two categories of patients: (1) those with intact memory function and a normal hippocampus ipsilateral to the seizure focus (“functional adequacy” hypothesis) and (2) those with ipsilateral hippocampal atrophy but impaired memory function, presumably related to poor function within the hemisphere contralateral to the seizure focus to be resected (“functional reserve” hypothesis).8,155 Patients with dominant-hemisphere TLE and a reversed Wada memory asymmetry score (i.e., better memory performance in the epileptogenic temporal lobe, with poor right temporal lobe performance) have been shown to have a greater risk for memory morbidity after left-sided resection, as well as poorer seizure outcome postoperatively.156 Patients with dominant-hemisphere hippocampal atrophy who undergo contralateral, non–dominant-hemisphere resections are also at risk for verbal memory deficits.157 Preoperative MRI studies of hippocampal volumes and left hippocampal MRS profiles (creatine/NAA ratio) also help predict the risk to verbal memory performance after surgery.62
In a recent study, a multivariate risk factor model for predicting postoperative decline in verbal memory was developed in which five risk factors were independently associated with outcome, including (1) dominant-hemisphere resection, (2) MRI findings other than exclusively ipsilateral MTS, (3) intact preoperative delayed recall verbal memory, (4) relatively poorer preoperative immediate recall verbal memory, and (5) intact ipsilateral memory performance on the Wada test.8,158 With this model, individual patients can be assessed with respect to their risk for deficits in verbal memory function after surgery.
Standard Anterior Temporal Lobectomy Versus Selective Amygdalohippocampectomy: Memory Outcome
In patients thought to be at risk for global or material-specific memory deficits postoperatively, various management strategies have been proposed to reduce these losses,8 including memory mapping in the temporal neocortex with restriction of neocortical resection, SAH, or simple denial of surgery to these patients.159 With reports of global amnesia occurring in patients undergoing SAH,149 it was thought that it may be advantageous to perform selective mesial resection from the standpoint of preservation of material-specific memory, particularly short-term verbal memory function. Although some early outcome studies in small series of patients suggested a possible advantage of SAH over standard anterior temporal lobectomy from the standpoint of postoperative memory outcome,88,160 this has not been supported by other studies, and there have been reports to the contrary.161
In a recent review of 140 patients undergoing either right or left SAH, a decline in verbal learning and memory occurred after 32% of the right-sided and 51% of the left-sided resections.54 The left SAH patients were particularly at risk when preoperative testing revealed intact verbal memory function, late onset of epilepsy, and the absence of MTS on MRI. Collateral damage to adjacent temporolateral tissue during the transsylvian dissection may exacerbate the deficits caused by hippocampal resection.54,162 The role of deafferentation of the temporal circuitry during resection of the parahippocampal gyrus, amygdala, and hippocampus also needs to be considered. This is supported by PET evidence of worsening hypometabolism of the remaining temporal lobe neocortex after SAH.163
Postoperative Language Dysfunction
After dominant-hemisphere temporal lobe resection, a syndrome of transitory postoperative dysnomia or even aphasia is observed in as many as 30% of operated patients.164 In most cases, the dysnomia or aphasia gradually disappears over a period of a few weeks. This occurs even when resections are guided by intraoperative or extraoperative language mapping.126,127 The cause of this transitory phenomenon is unclear, but it is more common when resections are carried to within 1 to 2 cm of essential language sites as determined by mapping procedures.8,159,165 Other explanations for this phenomenon include resection of inferior temporal lobe “inessential” language sites,166 brain retraction and associated “neuroparalytic edema,”167,168 or deafferentation of white matter pathways. Some authors have suggested that such word-finding deficits represent an acute postoperative exacerbation of the preoperative deficits common in patients with TLE and that they last no longer than 1 year.169
Although some investigations of naming have not revealed enduring deficits at 6 and 18 months postoperatively,170–172 others have suggested that significant, persistent word-finding difficulties do occur commonly after standard or anteromesial temporal lobe resection.164,173,174 Such deficits have been reported to be associated with early risk factors for the development of seizures173 and with the pathologic state of the resected hippocampus.175 In one study, 7% of patients undergoing standard dominant-hemisphere resections exhibited persistent postoperative dysnomia.174 Ojemann described enduring language deficits after resections within 1 to 2 cm of identified language sites.176
The aforementioned findings stimulated interest in the value of intraoperative mapping and tailoring of the lateral neocortical resection. Ojemann and colleagues suggested that up to 17% of patients undergoing left temporal resections 4.0 to 4.5 cm from the temporal tip (a “standard” temporal lobe resection) without mapping would experience postoperative deficits.8,177 Some centers now restrict cortical resections to 3 cm of the middle and inferior gyrus without mapping and have reported minimal postoperative language deficits.103 A general trend toward restricted lateral cortical resection in the temporal lobe has resulted in language mapping being less commonly used. It has not been studied whether such restricted resection may engender deficits not seen in patients undergoing mapping.
Persistent, severe dysphasia has been reported in 1% to 2% of patients undergoing dominant-hemisphere temporal resections, even with language mapping.116,143,178,179 Such adverse postoperative outcomes occur as a result of resection of essential language cortex or manipulation or thrombosis of the middle cerebral or anterior choroidal artery.180
Neurobehavioral and Psychosocial Outcomes
Psychiatric Outcome
Psychiatric morbidity has been reported to occur in 15% to 50% of patients with epilepsy in the literature.181,182 There is a high prevalence of psychopathology, including depression, in candidates for temporal lobe resection both preoperatively and postoperatively.183,184 One study reported postoperative improvement or resolution of long-standing depressive symptoms in 47% of patients undergoing temporal lobe resections, thus suggesting that preoperative depression is not a contraindication to surgery.185 In the same study, depression occurred de novo in 10% of operated patients. Improvement in depression postoperatively is more likely in patients who are rendered seizure free.186,187 Preoperative assessment of the risk for chronic depressive symptoms postoperatively may be achieved by using measures of emotional adjustment, such as the Washington Psychosocial Seizure Inventory.187 The early postoperative period is characterized by the dynamic expression of varying psychopathologic conditions. In one study, half of the patients with no psychopathology preoperatively exhibited symptoms of anxiety, depression, and emotional lability 6 weeks postoperatively.188 Other reports have documented new psychiatric problems in 31% of patients and resolution of psychiatric diagnoses in 15% of patients in the 6 months after surgery.183 One study reported that 10% of 121 patients with TLE who underwent epilepsy surgery required postoperative psychiatric hospitalization.189 The de novo appearance of hypomania requiring psychiatric hospitalization,190 psychogenic seizures (particularly in females undergoing nondominant temporal lobe surgery),183,191 and neurotic or psychotic symptoms192 postoperatively demonstrates the necessity for comprehensive psychosocial and psychiatric assessments both preoperatively and postoperatively. In the context of a thorough preoperative evaluation, a history of psychotic symptoms does not represent an absolute contraindication to surgical intervention, although an exacerbation of symptoms may occur postoperatively.8
Psychosocial Outcome
It is increasingly being recognized that the syndrome of intractable TLE embraces comorbid conditions beyond the encumbrance of frequent, intractable seizures. Such comorbidity includes psychosocial, psychiatric, and neuropsychological impairment, medication toxicity, and excess mortality rates.8 These impairments develop as a result of frequent, disabling seizures during critical stages of personal development and may not resolve immediately after surgery.
Patients are aware of epilepsy-associated disabilities and hope for their resolution after surgery. In a study of 69 preoperative patients, their aims for epilepsy surgery beyond freedom from seizures included desire for work, ability to drive, independence, socializing, and freedom from AEDs.9 The psychosocial outcomes of successful surgery were assessed in a 5-year follow-up study of the long-term changes in 61 surgical and 23 medically managed TLE patients.193 In this study, 68% of the surgery group exhibited improved psychosocial status, as opposed to 5% of the medically managed group. Individuals who underwent surgery were found to be more likely to drive, live independently, work full-time, and be financially independent. Remaining seizure free was not a prerequisite for improvement in psychosocial measures in this study, although other investigations have documented diminished psychosocial adjustment in patients with recurrent seizures.194
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree