Fig. 22.1
Most common origin sites of facial nerve schwannomas. ICA internal carotid artery, TN trigeminal nerve, TU tumor
22.2 Surgical Anatomy
The surgical anatomy of the petrous apex, temporal bone, CPA, and internal auditory canal in relationship to the facial nerve must be understood in order to choose the best surgical approach with minimal morbidity (Fig. 22.2). From the brain stem, the facial nerve runs into the internal acoustic meatus (meatal segment) to enter the facial canal within the petrous portion of the temporal bone. This portion of facial nerve named as labyrinthic portion has 3–5 mm [23] and contains the geniculate ganglion. The geniculate ganglion is anterior and medial to the arcuate eminence and gives the greater petrosal nerve as its main branch that runs anteriorly to the pterygopalatine fossa. In about 15 % of the cases, the temporal bone is dehiscent over the geniculate ganglion [25] and may be injured during surgical exposure of the petrous apex. The facial nerve continues its course inside the temporal bone through its tympanic portion and has laterally a close relationship with the tympanic membrane. The facial nerve exits the skull through the stylomastoid foramen and goes to the parotid gland running anteriorly to the digastric muscle. At the stylomastoid foramen, the facial nerve is identified using as landmarks the mastoid tip, the posterior belly of digastric muscle, and the cartilaginous portion of the external acoustic meatus. The superior trunk provides innervations to the orbital muscles (temporal and zygomatic branches). Other branches from the superior trunk are the buccal and mandibular nerves. The posterior auricular nerve, the digastric nerve, and the cervical nerve branch arise from the inferior trunk.
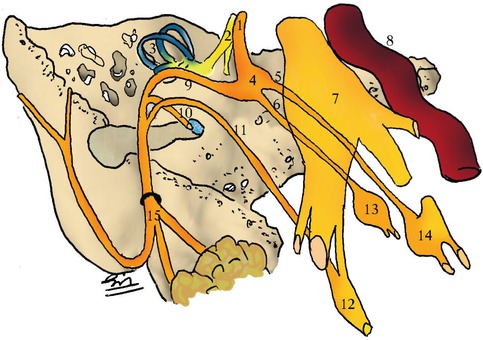

Fig. 22.2
Facial nerve anatomy. 1 intracanalicular portion, 2 cochlear nerve, 3 semi-circular channels, 4 geniculate ganglion, 5 Large superficial petrosal nerve, 6 small superficial petrosal nerve, 7 Gasserian ganglion, 8 internal carotid artery, 9 labyrhintic and mastoid portions, 10 branch to stapedius, 11 chorda tympani nerve, 12 submaxillary ganglion, 13 otic ganglion, 14 sphenopalatine ganglion, 15 extra-temporal portion
22.3 Diagnosis
22.3.1 Clinical Symptoms
The presentation of FNS tends to be insidious, manifesting with different clinical features depending on the location and extent of the lesion. Establishing a correct preoperative diagnosis of FNS is important to plan treatment and advise the patient. Facial palsy and hearing disturbances are the predominant presenting symptoms, but it depends on the location and extent of the lesion. It can be classified as intracranial, intratemporal, and extratemporal, with symptoms being different for each one [4].
Intracranial lesions (i.e., at the CPA) and those in the internal auditory canal frequently result in sensorineural hearing loss, tinnitus, and vestibular symptoms because of compressive effects on the eighth nerve. These tumors are commonly misdiagnosed as vestibular schwannomas. Intratemporal FNSs often present with facial palsy and with conductive hearing loss, which occurs as the tumor expands into the middle ear space.
According to Chung et al. [3], patients with intratemporal tumors showed more severe symptoms such as hearing loss, tinnitus, dizziness, and facial weakness or pain than patients with extratemporal FNS. Hearing loss is observed in patients with intratemporal tumors. According to its location, this loss may be sensorineural or conductive. Tumors in the labyrinthine segment, near the geniculate ganglion, can present sensorineural hearing loss. Conductive hearing loss is observed in tumors arising in the horizontal segment. Some patients can be totally asymptomatic [18, 19, 24]. Facial paresis is often slowly progressive and preceded by facial twitching [10, 21]. It occurs more frequently in patients with intratemporal tumors. Facial paresis in Bell’s palsy has a sudden onset often accompanied by periauricular pain and hyperacusis. FNS is the cause of paresis in about 5 % of patients with diagnosis of Bell’s palsy. Saito and Baxter [27] found an 84 % incidence of facial palsy in intratemporal tumors and 34 % in extratemporal tumors. Extracranial tumors usually present as parotid or retromandibular masses at the skull base [4]. Facial palsy is rare in these cases [6]. O’Donoghue et al. [18] found facial palsy in only 45 % of 48 reported cases. Preoperative differentiation of a small intracranial FNS from an intracanalicular vestibular schwannomas may be difficult especially if the patient has no facial nerve palsy. The correct diagnosis is done only during surgery, and the surgeon is faced with a difficult decision: remove the tumor, causing an unexpected facial palsy, or abort the procedure.
22.3.2 Radiological Findings
Imaging characteristics of a facial schwannoma itself are the same as that of schwannomas of other cranial nerves: homogeneous enhancement when small on both CT and MRI and heterogeneous when the tumor is large. Tumor extension along the facial nerve canal and the labyrinthine segment is the best signal of a facial nerve schwannoma rather than a vestibular schwannoma. There is a universal acceptable imaging description of these lesions as enhancing tubular masses (MRI) in a smoothly enlarged facial canal (CT). However, the term “jack of all trades” seems to be applicable for this classic description, since it has been described in the recent literature, in an almost definitive research on such subject by Wigins III et al. [32], with at least five other possible imaging appearances for these entities. Stasolla et al. [30] have described an FNS with cisternal and intracanalicular components extending to the geniculate ganglion through an enlarged labyrinthine segment of the fallopian canal configuring an important sign: “the labyrinthine tail.” On coronal plane, this tumor featured a short but clear “dural tail” emphasizing the lack of specificity of this sign, mostly often related to meningiomas.
22.3.2.1 Imaging Aspects
On MRI, the classic description of this tumor is a well-circumscribed fusiform enhancing mass along the course of intratemporal facial nerve on T1-enhanced studies. On CT with bone algorithm sharply, canal enlargement is observed. These statements are not enough to encompass the whole possible imaging appearances regarding these tumors. Modern imaging techniques are capable to better depict the effects on the surrounding anatomic landmarks related to these lesions and offer not only the correct diagnosis but a better surgical planning. Regardless of anatomic location, schwannomas presented as heterogeneously or homogeneously enhancing soft tissue attenuation isoattenuated to gray matter on enhancing CT and may present cystic foci. In the temporal bone, FNS will depict benign-appearing scalloping and remodeling of surrounding bony structures. It is not true erosion, but rather a more benign osseous expansion. MRI can show heterogeneity within large FNS and predominant hyperintensity on T2 sequences and iso- to hypointensity on T1-weighted series. Homogeneous enhancement is the rule on post-contrast-enhanced MRI (better demonstrated with fat-suppression techniques, especially concerning intracanalicular and intratemporal lesions). Larger lesions may undergo cystic degeneration. Special attention should be paid to the increasing use of 3D CISS sequences (contrast interference with steady state). These sequences are sub-millimetric T2-weighted acquisitions that better depict CSF-immersed structures mostly cranial nerves and anatomic landscapes in the vicinity (or in interface of) CSF. There is a good conspicuity of cisternal, meatal segments of the seventh and eighth cranial nerves, internal auditory canal, and its fundus as well as the cochlea and vestibule when using this type of sequence. Its usefulness has been proved and now makes part of our imaging protocol at our institution when facing patients with vertigo, facial palsy, and hearing loss among several other indications.
22.3.2.2 Radiological Findings According to Site of Origin
A dumbbell-shaped tumor may be present if there is an extension from the internal auditory canal through the labyrinthic segment of the facial canal into the geniculate fossa (Fig. 22.3). This aspect associated with T1 MRI enhancing sharply scalloped or fusiform enlargement of the facial nerve canal is diagnostic of FNS. It should be, however, differentiated from a transmodiolar acoustic schwannoma when there is extension into the cochlea through the modiolus in a cochlear nerve schwannoma (Fig. 22.3). When the geniculate fossa is involved primarily or there is involvement of the greater superficial petrosal nerve, the appearance is distinct. It seems like an extra-axial middle cranial fossa mass (Fig. 22.4). This situation claims for an inspection of the geniculate ganglion or the greater superficial petrosal nerve to search possible sites of origin. When the geniculate ganglion is affected, there is a “bulbous” appearance of the middle cranial fossa. FNSs originating in the greater superficial petrosal nerve scallop the anterior margin ganglion and adjacent bony petrous apex. The FNSs involving the tympanic segment of the facial nerve have different morphologies and usually are multilobular instead of the classic fusiform appearance since in this segment bony structure is more fragile. A fistula to the semicircular canal may develop if there is medial or superior extension. Inferior and lateral extension can cause lateral displacement of the ossicular chain and lead to conductive hearing loss.
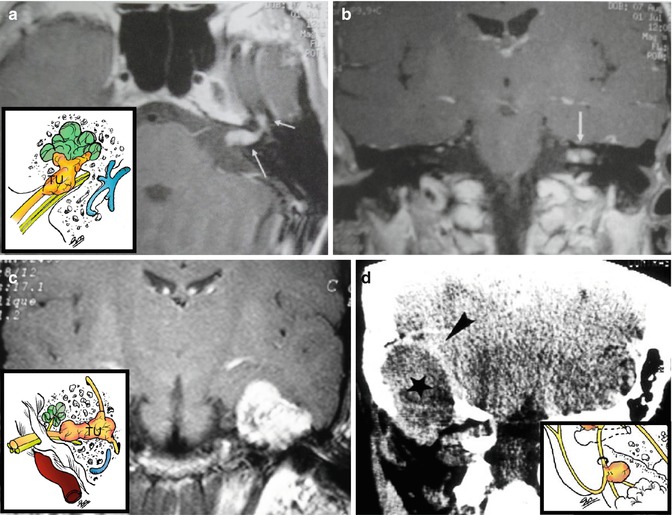
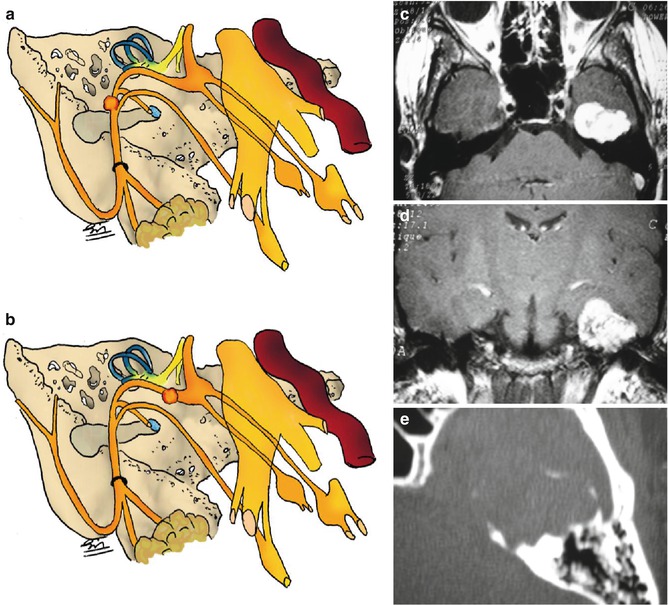

Fig. 22.3
(a) Intracanalicular facial schwannoma (arrows). (b) Vestibular Schwannoma (arrow). (c) Tumor in the geniculate ganglion. (d) CT: Extra-temporal schwannoma (asterisk) elevating the dura mater (arrowhead)

Fig. 22.4
(a, b) Drawings showing tumor site within the temporal bone. (c, d) MRI with Gd showing intratemporal tumor. (e) CT scan demonstrating petrous bone erosion and calcification
The appearance of an FNS involving the mastoid segment is quite distinctive. In this segment, the facial nerve canal is surrounded by delicate thin-walled bony septations separating air cells, and expansion of the tumor can result in an aggressive aspect due to a breakthrough into this aerated cells; without bearing this context in mind, misdiagnosis is possible and should raise a possibility of facial nerve hemangioma or other locally invasive entity.
22.3.3 Differential Diagnosis
Differential diagnostic considerations regarding FNS include cholesteatoma, facial nerve hemangioma, and perineural parotid malignancy. Intratemporal facial nerve enlargement is present in these entities. Congenital or acquired cholesteatoma involving facial nerve canal does not enhance on T1-enhanced MRI. Facial nerve hemangioma of facial nerve canal and adjacent bone presents more aggressive aspect with irregular margins and may have even a “mouth-eaten” appearance. Ossifying fibroma can present a calcified honeycomb matrix. A parotid mass may spread its malignancy from distal to proximal along an enlarged facial nerve canal. Idiopathic Bell’s palsy is another differential diagnostic consideration that should be paid attention. Indeed, some patients, with mild facial nerve palsy, can receive this diagnosis. The typical Bell’s palsy patient usually presents an enhancing “tuft” in the internal auditory canal fundus plus enhancement of all or part of the facial nerve. Bell’s palsy patients, however, do not present an enlarged facial nerve canal on CT, and this feature can, indeed, separate these two entities. When involving the CPA-IAC, FNS can be indistinguishable from acoustic schwannomas if there is no extension through labyrinthine segment of the facial nerve. Because of that, recently diagnosed acoustic schwannomas should be inspected for a “labyrinthine tail” since the presenting symptoms will not always suggest the site of origin of the tumor.
22.4 Current Management
The decision on how to treat these patients, the timing of intervention, and the surgical approach should be individualized, depending on the extent of the tumor, grade of facial palsy, hearing function, surgical experience, and informed patient consent. One should bear in mind that complete tumor resection is curative; however, the best postoperative facial function obtained after tumor resection and nerve reconstruction is a House-Brackmann (H-B) grade III palsy. When moderate to total facial palsy is already present and for large tumors in the cerebellopontine angle compressing the brain stem, complete resection is indicated [1]. Planning surgery should include detailed explanation to patients, presenting the rationale behind the timing of intervention and the alternative management (conservative and radiosurgery). Complete tumor removal should include proximal and distal nerve margin verified by frozen section. Nerve graft donor site (greater auricular or sural nerve) is prepared. Hypoglossal/facial anastomosis is indicated only if a proximal stump cannot be found. Tumor removal preserving the nerve has been described in small FNS, but in the majority of cases the nerve must be transected. Postoperative eye protection measures (tarsorrhaphy or implant of gold weight) are anticipated. Pure tone audiogram, electroneuronography, facial nerve action potential, and photography of patients face are measures for assessing facial and cochlear nerve preoperative function making postoperative comparison possible.
Nowadays, the primary goal is to extend optimal facial nerve function [15, 33]. Liu and Fagan [11] treated ten patients conservatively. Eight patients maintained normal facial function for up to 10 years after presentation, and no significant tumor growth was observed on serial radiological imaging control. Thus, the management of FNS has evolved from predominantly resection and grafting to increased use of more conservative approaches that might be called “facial nerve preservation” approaches.
These include observation with serial MRI imaging, bony decompression, and stereotactic radiation [33].
22.4.1 Conservative Surgical Approach
Decompression for intratemporal FNS involves widening of the narrow confines of the fallopian canal without tumor removal. It was first reported in 1997 by Angeli and Brackmann [1]. Four patients were operated on and postoperatively presented with facial function House-Brackmann grade I or II. In the follow-up averaged 45 months, the patients maintained excellent facial nerve function, and there was little to no tumor growth based on serial imaging. Nerve-preserving stripping surgery was first reported by Lee et al. [9], who had adopted it since 1995 in all patients with preoperative facial nerve function better than H-B grade III. The technique involves removing the schwannoma from the remaining nerve fascicle. The junction between the nerve and the tumor capsule is identified; the nerve is meticulously dissected from the tumor, which is then removed through careful intracapsular piecemeal bulk reduction without injury to the neural fascicle [8]. At the final functional assessment, 7 patients had no change in facial function, 2 improved, and 6 worsened. Specifically, 4 patients had normal facial function, 8 had H-B grade II, and 3 had H-B grade III.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








