Chapter 59 Fetal Surgery for Open Neural Tube Defects
The goal of fetal surgery is to prevent or reduce the adverse consequences of a congenital disorder without increasing the risks for the fetus and mother. A number of diseases, such as congenital diaphragmatic hernia and sacrococcygeal teratoma, can be treated by surgical procedures performed directly on the fetus prior to the anticipated delivery date.1 An open neural tube defect, or myelomeningocele, is different in that it is not a fatal disease and fetuses with this condition will usually complete a normal gestation.2 This is an ethical challenge for the treating physicians because the potential benefits must be balanced by a well-defined risk to the fetus, and also to the mother who is a “bystander” with respect to the perceived benefits.
The development of fetal surgery for a specific condition has usually moved in a series of steps starting from the development of an animal model, definition of the natural history, refinement of techniques in test cases, and finally, evaluation of efficacy in prospective clinical trial. The reported benefits of fetal surgery for myelomeningocele as determined from retrospective case series include a reduction in shunt insertion rates and improvement in the hindbrain abnormality.3–5 To obtain more conclusive data, the potential benefit of fetal surgery for myelomeningoceles is being examined in a clinical trial directly comparing patients randomized into prenatal and postnatal treatment groups.
Rationale for Fetal Repair
Although there is large spectrum of abnormalities observed in children with myelomeningoceles, it is useful to separate the neurologic deficits into two groups: primary and secondary. The primary neurologic deficits are those directly caused by the arrested development of the neural placode, which usually occurs in the lumbosacral region.6 Because neural tube closure occurs during the third and fourth weeks of gestation, the spinal cord in this region is very immature at the stage when a myelomeningocele develops. Although the structure of the spinal cord is severely disrupted at the involved level, it is unknown whether the placode is capable of further development.7 The functional neurologic level is either at the same level as the vertebral anomaly, or actually higher than the vertebral level, resulting in worse neurologic function, in more than 80% of patients with open forms of spina bifida.8
The theoretical advantage of fetal repair for myelomeningoceles is that the neural tube is covered and protected many months before the expected delivery date. The basis for expecting improved neurologic function is that restoration of the dysplastic neural placode within the spinal canal isolates it from the amniotic fluid and prevents ongoing injury.9,10 Mueli and others surgically created a spinal-cord lesion in fetal sheep at 75 days of gestation that simulated a spina bifida lesion.11 After delivery at term, the gross and microscopic appearance of the exposed spinal cord resembled a human spina bifida lesion and the animals were incontinent and had loss of sensation and motor function below the lesion level. One group of animals with surgically created spina bifida lesions were then treated using a myocutaneous flap at 100 days of gestation. These animals were then carried to full-term gestation and had near-normal motor function and normal bowel and bladder control. The results of these experiments suggested that early repair of an exposed spinal cord may preserve neurologic function and may allow improvement through plasticity.12 Although provocative, these large animal experiments clearly rely on a model system that has distinct differences with the human disease.
Timing for Fetal Surgery
If closure of an open neural tube defect reduces secondary injury occurring to the placode, then surgical intervention should be performed as early as possible. In practice, surgical timing is determined by diagnosis and technical limitations of the actual procedure. Most myelomeningoceles are detected during the second trimester, either during an investigation of a positive maternal screening test, or during a routine ultrasound study. The quality of current ultrasonography allows detection of most fetuses with myelomeningoceles by the mid-portion of the second trimester.13 From a practical viewpoint, this means that a diagnosis is made between 18 and 22 weeks of gestation. Taking into consideration current obstetrical practice, it is unlikely that detection of fetuses with spina bifida will occur any earlier unless new, more sensitive screening tests are discovered.
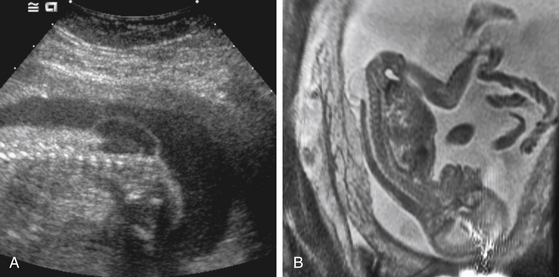
Preoperative fetal imaging studies usually begin with a detailed ultrasonogram (Fig. 59-1A). This study is able to determine some anatomic features with great precision. These include size of the overlying sac, the level of the defect, the position of the cerebellar tonsils, and the presence of lower-extremity deformities. Limitations include difficulty determining some associated brain anomalies, other intraspinal anomalies, and at times, the exact dysraphic level. Most patients being considered for fetal surgery will undergo a fetal MRI study (Fig. 59-1B). Because of motion, images in sagittal, axial, and coronal planes are obtained randomly by repeatedly imaging the fetus over time. The preferred MRI technique is a single-shot, fast spin-echo T2-weighted sequence. There is some evidence that MRI may improve the ability to detect coexisting spinal and brain anomalies that may not be apparent on ultrasound studies.14,15
Hysterotomy and Exposure
Several technical hurdles needed to be overcome before fetal surgery could be performed safely. These include: (1) the ability to open the uterus and prevent separation of the chorioamniotic membranes, (2) achieve watertight uterine closure, and (3) prevent the onsent of preterm labor in the post-operative period.1 The ability of the mother to carry and deliver subsequent pregnancies does not appear to be jeopardized by fetal surgery.