(1)
Division of Critical Care Neurology, Mayo Clinic, Rochester, Minnesota, USA
Abstract
This chapter introduces 12 landmark trials that define the field. These clinical trials involve the first testing of drugs in large groups of patients and modified or altered practice.
Short Historical Note 40
Triple H: Hypertension–Hypervolemia–Hemodilution Therapy
The Title of the Paper
Kosnik EJ, Hunt WE. Postoperative hypertension and management of patients with intracranial arterial aneurysms. J Neurosurg. 1976;45:148–54.

Fig. 6.1
Title page
The Paper and the Times
In the late 1940s, it became clear that cerebral infarcts could occur after a ruptured aneurysm, and in particular situated remotely from the aneurysm. Pressure from hematomas, thrombosis and embolism, hypotension, and arterial vasospasm were considered mechanisms [124]. Decades later, when neurosurgeons tried to grasp the consequences of aneurysmal rupture, the development of cerebral vasospasm was recognized as a major cause of deterioration [67]. The mechanism of cerebral vasospasm remained unsatisfactorily explained, and experimental studies testing the effects of “spasmolytic” drugs in animal models also were not encouraging. Different approaches to treat this complication were considered, and they involved manipulating the blood rheology and arterial pressure.
Thus, in cerebral arteries without autoregulation, increasing cerebral blood flow (CBF) could be achieved by increasing cerebral perfusion pressure (CPP) or by reducing viscosity. This concept became the basis of hypertension, hypervolemia, and hemodilution therapy, better known as “triple-H” therapy.
Neurosurgeons Kosnik and Hunt were the first to describe the use of postoperative hypertension, but in fact, they used a combination of volume augmentation and hypertension. No experimental study had previously investigated the possible effects of this intervention, and experience came directly from clinical observations.
The Details of the Paper
The paper was based on the assumption that increasing the CPP would be the most effective way to overcome the severe and diffuse cerebral vasospasm in these patients (Figs. 6.1). The authors describe seven patients with an aneurysmal subarachnoid hemorrhage (SAH), some with postoperative neurologic signs, and others with a neurologic decline while the aneurysm was unsecured. The patients did well until postoperative day 4 or 5, and then became markedly “obtunded with only semi-purposeful movements in response to pain.” Patients were treated with colloids and plasmanate followed by norepinephrine. Norepinephrine was used because of both its powerful peripheral vasoconstriction and its inotropic stimulating effects on cardiac muscle. The investigators developed a regimen that they described as “enough norepinephrine to elevate the blood pressure 40–60 points systolic or to produce unmistakable clinical improvement.”
Additionally, they administered plasma followed by whole blood transfusion. After blood volume expansion and observing a rise of the central venous pressure, norepinephrine was weaned to a lower dose. After the neurologic deficit improved, blood pressure was maintained for several more days and then gradually allowed to drift down to pretreatment level.
Equally important, they noted that a reduced blood volume could have been present in some of their patients because there was a rapid response with volume augmentation. Additionally, in one patient with adequate systolic blood pressures in the 140 mmHg range, further blood pressure augmentation was successful, suggesting an unknown ceiling of blood pressure effect. The authors also emphasized the importance of timing of augmentation. “The most serious hazard seems to be the institution of treatment too late, after the cerebrovascular system is so damaged that increasing perfusion pressure only increases brain swelling.”
The authors recognized the potential hazards of increasing perfusion that could potentially result in brain edema. There also was a concern that fluid overload in patients with congestive heart failure could further complicate management.
The Message and Acceptance
In literature reviews, two other important manuscripts are usually mentioned concurrently. One paper by Pritz et al. in 1978 described intravascular volume expansion in four additional patients, and their approach was similar, keeping the patient hypervolemic and additionally using vasopressors or discontinuation of antihypertensive medication (Figs. 6.2 and 6.3) [116]. In 1982, Kassel et al. published their surefooted data on 58 patients who deteriorated neurologically from angiographically confirmed cerebral vasospasm. In this study, 81% of the patients responded favorably to therapy, and failure in the remainder of the patients was related to delay initiating therapy or to development of complications, particularly pulmonary edema from overhydration [80] (Fig. 6.4).
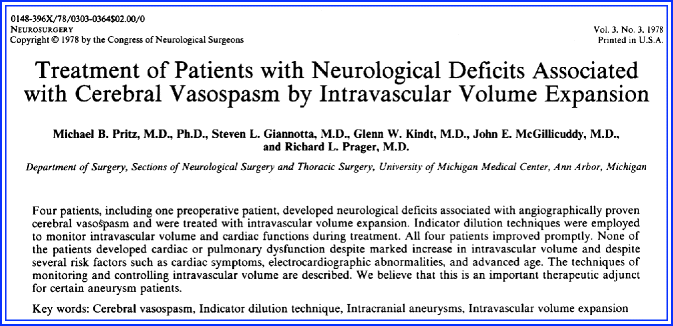

Fig. 6.2
Title page

Fig. 6.3
Pritz’s patient example

Fig. 6.4
Title page
The papers tackled the major issues of timing and target of blood pressure augmentation. Kassel’s study was unique because for the first time it introduced Swan-Ganz catheterization to monitor intravascular volume and this approach—until recently—became universal in hemodynamic augmentation of patients with SAH complicated by vasospasm.
Complications of aggressive monitoring also became apparent. One study found catheter-related sepsis in 13%, but less commonly cardiac failure (2%), subclavian vein thrombosis (1%) or pneumothorax (1%) [92].
One other important question emerged. Could prophylactic triple-H therapy—instituted before the onset of symptoms—be more effective?
Solomon et al. studied 47 consecutive patients with ruptured intracranial aneurysms that were treated with prophylactic volume expansion for up to 2 weeks after SAH and found no cases of cerebral infarction. However, another key study revealed no difference in mean global CBF—using 133 xenon clearance—in hypervolemic and normovolemic patients. Symptomatic vasospasm occurred in 20% of patients in each group, despite better intravascular volume parameters [135, 136].
The effect of 5% albumin solution on sodium balance and blood volume after SAH was studied in 43 patients treated with either hypervolemia or normovolemia for 1 week after aneurysm clipping. The hypervolemia group received significantly more total fluids, sodium, and 5% albumin than did the normovolemia group and had higher central venous pressure levels. Supplemental 5% albumin solution prevented sodium and fluid losses but did not have an impact on blood volume [95]. Finally, one study found that an increase in cardiac output without changes in mean arterial pressure can increase CBF in the setting of vasospasm and suggested dobutamine as a first measure [78].
Current published data on the best approach are unconvincing because consistent measurements of relevant clinical and laboratory variables are lacking, with different methods used in each of the cohorts. A randomized blinded study of hemodynamic augmentation in aneurysmal SAH has never been performed. Nonetheless, many neurointensivists and neurosurgeons have seen significant improvement of the patient with aggressive fluid management, and supplemental blood pressure increase started soon after onset of symptoms [90]. But when a patient deteriorates, management now has shifted toward early endovascular management in combination with volume augmentation and vasopressors.
Short Historical Note 41
Treatment of Status Epilepticus
The Title of the Paper
Whitty GWM, Taylor M. Treatment of status epilepticus. Lancet. 1949;2:591–4.

Fig. 6.5
The title page
The Paper and the Times
Status epilepticus (SE) was known by the ancient Greek physicians; however, its severity was not always recognized as such, and there was widespread admission to asylums even up to nineteenth century Victorian times [16]. According to Neligan and Shorvon, the first mention of “status epilepticus” was in a translation of Trousseau’s classic book on clinical medicine, Clinique Médicale de l’Hôtel-Dieu de Paris, 1868 [101].
In the twentieth century in Europe, Bourneville, Westphal, Charcot, and Browne were among physicians who described SE in sufficient detail and often treated it with large doses of Bromides [97, 133]. In the United Kingdom, work done at the National Hospital for the Paralyzed and the Epileptic Queen Square in London — with most notable Gowers as a leading contributor — further elaborated on how to treat SE [57].
Many physicians recognized that once the diagnosis was established, the prognosis could be poor. One of the first descriptive works on SE is by Clark and Prout [31]. They defined SE as a “maximum development” of epilepsy with one seizure after another and with coma in between these seizures. They also recognized tachypnea, tachycardia, and hyperthermia as manifestations. Clark and Prout also purported to show destruction in cortical layers and a relationship with duration of SE, with more severe abnormalities with longer episodes of seizures. This has been considered one of the first documents that emphasized the severity and consequences of this condition and was a novel insight at the time. SE became recognized as following several stages: premonitory, convulsive, and comatose stages [132].
The history of treatment of SE was recently comprehensively summarized by two experts in the field [97, 101]. Before the turn of the century, chloral hydrate and ether were considered for frequent seizures. Phenobarbital was introduced in 1912 [109], paraldehyde in 1914 [34], chloroform anesthesia in 1928 [115], and phenytoin in 1958 [26].
SE—then and now—occurred more often in patients with prior epilepsy. It was also customary at the time to point toward sudden withdrawal of bromides or phenobarbital as a cause of SE. Gradually, it also became quite clear that outcome of the condition could reflect the disorder causing SE in the first place and also that time to resolution of SE was an important factor.
The Details of the Paper
The study involved 36 patients with SE at Radcliffe Infirmary or the head-injury bureau at Wheatley Military Hospital. Seventeen of 36 patients were seen by the authors. The authors emphasized “this condition constitutes a grave medical emergency and may be fatal unless the fits are rapidly controlled.” They found evidence that paraldehyde was the drug of choice both because it rapidly controlled seizures and was successful even when barbiturates failed to treat the neurologic emergency. The authors suggested a protocol for the first time.
As soon as possible 8–10 mL of paraldehyde is injected into the gluteal muscle, and the site of injection is massaged. The paraldehyde need not be sterilised. This treatment usually stops the fits within half an hour.
If the fits continue, 5 mL of paraldehyde intramuscularly is given every half-hour until they cease. The persistence of focal twitching without any tendency to spread does not require further sedation, and attempts to eliminate these entirely may lead to a dangerous level of narcosis.
If the patient’s general condition indicates it, an intravenous glucose-saline or plasma drip is given at the rate of one bottle in 3 h. This will also provide a convenient method of continuing the administration of paraldehyde, either by intermittent injections into the drip tubing, or in solution in the drip fluid in any required concentration, since it is soluble 1 in 8 in physiological saline solution.
They also suggested that the patient should be placed on regular anticonvulsant therapy—barbiturates and hydantoins; and anesthetics were described.
If phenobarbitone is used it should be given in doses of 6–12 gr. smaller doses being of little value, and preferably in the “soluble” form by intramuscular or intravenous route. Thiopentone in anesthetic doses may be used, or chloroform anesthesia if no other remedy is at hand. But the effect of these methods is often short-lived, and it may be necessary to establish heavy sedation by other means and to maintain this for twenty-four hours or longer afterwards if the fits tend to recur.
The underlying theme of the paper was that prognosis of SE—in particular the fatal cases—was directly related to the start of treatment. Uncontrolled seizures or late treatment led to a higher probability of fatal outcome. Moreover, the emphasis was on paraldehyde as an important first drug, and they suggested a treatment algorithm—a novel approach to seizures and SE in particular.
The Message and Acceptance
In SE, well defined instructions to manage a persistently seizing patient are helpful, if not essential. Thus, algorithms have dominated the literature on SE, and with more experience, a reasonable acceptable practice evolved. Questions can be raised about initial treatment, which drug and dose, and how to manage refractory SE. Most physicians have now accepted that the first line of treatment should include a combination of a benzodiazepine (i.e., lorazepam or clonazepam) and phenytoin (or fosphenytoin) [159]. Others have advocated the initial use of short-acting midazolam, arguing that when it fails it does not linger [160]. Intramuscular midazolam was effective for prehospital status epilepticus treatment [134]. Second-line drugs have included phenobarbital, midazolam, propofol, valproic acid and there is little consensus among neurologists. How many physicians use a protocol is not known. When asked, many are not even aware of a hospital protocol [158]. Wasterlain and Treiman, in their monograph on SE, wrote a summary of the future (“The status of status epilepticus gives me fits”) and emphasized the “snail-like” progress in managing the disorder [160].
Only one major double-blind study [148] has been published in hospitalized patients and thus most of the agents currently used for SE did not go through rigorous efficacy and safety testing. Experiences with newer approaches continue to remain anecdotal (electroconvulsive therapy, hypothermia, levetiracetam), but comparative studies are very difficult to organize. SE has always been major neurocritical illness and not simply a severe manifestation of seizures. Not only is seizure control needed, but control of systemic manifestations is as urgent [28, 29, 115, 147].
Short Historical Note 42
Hemicraniectomy
The Title of the Paper
Rengachary SS, Batnitzky S, Morantz RA, Arjunan K, Jeffries B. Hemicraniectomy for acute massive cerebral infarction. Neurosurgery. 1981;8:321–8.
Fig. 6.6
Title page
The Paper and the Times
In ancient times, trepanation—sometimes of considerable size—was performed without a medical objective and more as a result of folkloric belief. It was reintroduced in the 1800s as a treatment for epilepsy.
Decompressive surgery—actually removing bone flaps—developed after the pathophysiology and neuropathology of increased intracranial pressure (ICP) became better understood. Kocher can be credited as one of the first physicians to explain its rationale [85]. Clinical experience may have started with Cushing’s paper on decompression in inaccessible brain tumors [36].
In some patients, decompressive surgery was performed with the assumption that the patient harbored a brain tumor, only to find there was a swollen hemispheric infarct [131, 137]. Decompressive surgery was also a last resort in traumatic brain swelling, but the earlier studies reported a circumferential approach—unsuccessful due to damage to the sagittal sinus and upward brain herniation—or a temporal decompression approach with small bone flaps [30].
In the 1970s, decompressive craniectomy—this time involving a large part of the skull—became again of interest to neurosurgeons, and experimental studies of posttraumatic cerebral edema showed survival of animals because the brain could swell through the opening [99]. Hemicraniectomy also was used by some groups in deteriorated patients with a large subdural hematoma who presented with extensor posturing and a dilated pupil. The development of a tight and bulging craniectomy site for a period up to 2 weeks was proof that the surgical decompression was justified. The chance of survival was 40% [122]. Furthermore, radical craniectomy also had been used in patients with severe brain swelling resulting from Reye’s syndrome.
Hemicraniectomy again went out of favor, but in the early 1980s was reintroduced as a primary treatment of ischemic brain swelling. Massive acute cerebral infarction—in particular in patients with acute carotid artery occlusion—was considered invariably fatal once cerebral edema developed. Anti-edema agents were rarely successful, and opening of the skull seemed the only option for many patients.
The Details of the Paper
A craniectomy to allow swelling outside the large opening was described. Decompressive craniectomy involved removal of a large bone flap and duraplasty. Generally, the swollen infarct was left intact because removal of necrotic tissue could cause major difficulty with hemostasis. All treated patients were young (ages 15, 27, and 51 years). Patients had a typical deterioration with uncal herniation (unilateral fixed pupil) signs in all three. Outcome was considerable with disability due to hemiplegia in all of the three treated patients. The authors considered the procedure for young patients, stroke involving the nondominant hemisphere, and absence of major comorbidity such as uncontrolled diabetes mellitus or dementing illness, and most importantly, the attitude of the family members to “accept attempts at preservation of life in the face of a severe neurologic deficit.” Decompression without temporal lobectomy was considered the best approach.
The Message and Acceptance
The procedure became rarely used in the United States, probably because few neurologists would consider the option in ischemic stroke.
Led by Werner Hacke, the group in Heidelberg reported success in a selected group of patients and further pioneered the procedure. The experience in initially more than 60 surgically treated patients, suggested that mortality is substantially decreased, but still leaving one of four patients with a severe disability. The outcome was poor from aphasia or neglect in addition to a severe hemiparesis [60].
There is now sufficient data that urgently salvaged patients who underwent this dramatic procedure actually may do well despite a fixed hemiparesis. Early decompressive hemicraniectomy (arbitrarily defined as surgery before the first signs of herniation) has been advocated, but under those clinical circumstances, a good result also can be explained by a favorable natural history. Concerns about major morbidity in survivors have been voiced, and the functional outcome in elderly patients is marginal. One study found severe disability more often in patients older than 55 years [118].
The discussion now pertains to selection of patients, timing (early vs. late), age (>55 years), dominance of involved hemisphere and comorbidity [81, 87, 94, 150, 163]. The procedure commonly is refused by family members and mostly performed in younger individuals. Many younger patients, however, eventually become independent [2].
Surgical technique of decompression after traumatic head injury now involves bifrontoparietal decompression [35]. Decompressive craniectomy is more commonly considered in patients with penetrating injury and considered more or less essential to preempt the commonly anticipated cerebral swelling. Complications associated with decompressive craniectomy after traumatic head injury and subsequent cranioplasty are subdural hygroma, relentless progression of contusions in up to 50% of cases, and hydrocephalus in 25% [35].
A new randomized trial performed by Australian New Zealand Intensive Care Society Clinical Trial Group found that outcome was worse in patients treated with decompressive craniectomy [35]. Patients were randomized when their ICP was greater than 20 mmHg after first-tier therapies. Patients in the decompressive surgery group had worse extended Glasgow Outcome Scale scores. Nonetheless, the surgery did reduce ICP and resulted in shorter stay in the intensive care unit. Therefore, there was a beneficiary short-term effect, but worse long-term outcome. The trial has been criticized as being overly aggressive because the design only required increased ICP for more than 15 min despite medical therapy. Many patients had ICP less than 20 mmHg, questioning the “refractory” designation. Moreover, the patients who underwent craniotomy had twice more common fixed pupils than the medically treated group, suggesting a worse neurologic state in the surgically treated patients. Another study—the Randomized Evaluation of Surgery with Craniectomy for Uncontrollable Elevation of ICP (RESCUE icp) trial—is under way. In this trial, bilateral craniectomy is considered only after an ICP threshold of 25 mmHg is reached for 1–2 h.
Decompressive craniectomy remains an important procedure. More room under the skull will lead to less damaging mass effect of brain swelling whether caused by trauma, penetrating injury, stroke, or an infectious process.
Short Historical Note 43
Factor VII for Cerebral Hemorrhage
The Title of the Paper
Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–85.
Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37.

Fig. 6.7
Title page

Fig. 6.8
Title page
The Paper and the Times
For many years, radiologists—while performing early cerebral angiography in patients with cerebral hematomas—had noted contrast extravasation indicative of continued bleeding. Prior to this observation, a cerebral hemorrhage—without an acquired coagulopathy or anticoagulation treatment—was considered a monophasic event.
Kelley and associates documented for the first time, enlargement of hypertensive hemorrhages [82]. They found significant hypertension (diastolic blood pressures of 140 mmHg) in these patients and considered it a possible factor.
Brott et al. studied 103 patients with computed tomography (CT) scans performed 2–6 h after onset and found 38% of the patients had more than a one-third growth in size within 1 h of admission to the emergency department (mean hematoma volume 26 mL of on initial CT and 32 mL on second CT). Deterioration was captured with scores from the National Institutes of Health Stroke Scale or Glasgow Coma Scale (GCS), and it was noted that not all patients deteriorated, including patients with significant increase in volume [21].
Nevertheless, several studies did document a correlation of outcome with clot volume, with some even suggesting that intracerebral hemorrhage volume of more than 80 mL had a 100% mortality despite intervention [20].
For several years, treatment for cerebral hematoma has remained inadequately studied with mainly a focus on the need for neurosurgical intervention. Blood pressure control also was considered uncertain, with many physicians interpreting a hypertensive surge as a compensatory response. It was argued that aggressive treatment of hypertension could result in worsening ischemia in the perihematoma penumbra.
Efforts to physically control the bleeding started with a pilot study of ε-aminocaproic acid but found continuous bleeding when administered within 12 h of intracerebral hemorrhage [112]. With the availability of recombinant activated factor VII (rFVIIa), it became logical to also study this drug. rFVIIa promotes hemostasis, and through this mechanism it could limit hematoma enlargement. Theoretically, these drugs also could accelerate thrombosis of bleeding, penetrating arteries or arterioles. Two trials were conducted in centers in China, Europe, and United States.
The Details of the Paper
The first rFVIIa trial randomized 399 patients within 3 h of onset into placebo, 40, 80, or 160 mcg/kg within 1 h of admission (Fig. 6.7). Patients who were admitted comatose (GCS score, 3–5), patients with planned surgery within 24 h, patients without spontaneous cerebral hemorrhage, patients on anticoagulation, or patients with known thrombocytopenia among other confounders were excluded. CT scanning was repeated 24 and 72 h after study treatment.
The first trial improved mortality by 38% with the higher (80 and 160 mcg/kg) doses. The reduction in hemorrhage size was stated as follows: “Treatment with rFVIIa resulted in reduced growth in the volume of intracerebral hemorrhage as compared with placebo by approximately 5 ml at 24 h, which translated into a 11-ml reduction in total lesion volume at 72 h.” Serious arterial thromboembolic adverse events were found more significantly than in placebo and in the higher doses.
The second clinical trial (Fig. 6.8), FAST (Factor Seven for Acute Hemorrhagic Stroke Trial), initially assigned 841 patients into placebo, 20 or 80 mcg/kg of rFVII within 4 h after onset. Several patients were lost to follow-up, and eventually the analysis included 262 patients who received placebo, 264 patients who received 20 mcg/kg, and 293 received 80 mcg/kg of rfVIIa.
This trial with fairly similar characteristics and execution as the prior trial found no improvement in outcome at 90 days.
A similar hemostatic effect of the drug was found with reduction of 29% in the placebo group to 14% in treated patients. The subgroup analysis suggested potential benefit for patients younger than age 70 years, with baseline hematoma volume less than 60 mL, baseline intraventricular hemorrhage volume less than 5 mL, and time from onset less than or equal to 2.5 h.
The Message and Acceptance
The discrepant results between the two studies demanded an explanation, and some differences were immediately evident. First, a higher incidence of intraventricular hemorrhage was present in patients treated with rFVIIa than in patients receiving placebo in the FAST study. Second, there was an increase in arterial thromboembolic events in patients receiving rFVIIa. Third, patients older than age 80 years were included, as were patients with large CT volumes (>60 mL) [42]. There was also a better than expected outcome in the placebo arm of the FAST trial. Tuhrim, in an editorial, noted two lessons that could be learned from these trials:
First, doses of rFVIIa larger than 80 mcg per kilogram increase the risk of arterial thrombotic events without a corresponding improvement in retarding hematoma growth; lower doses are not as effective. Second, randomization cannot ensure balanced groups, especially in trials of moderate size, and this in turn may affect results. Perhaps most important, these results emphasize two other principles that have emerged from other trials of stroke treatments: a single treatment approach may accomplish its physiological goal but may be insufficient to produce clinical benefit, and an intervention may be helpful to a well-defined subgroup but not to all those who have a particular disease [149].
Enthusiasm for these hemostatic drugs in nonanticoagulation-associated cerebral hemorrhages quickly waned. Hemostatic drugs such as prothrombin complex also became more popular as a result of low cost, longer action, and possibly fewer thromboembolic complications. Other avenues of treatment with rFVIIa were considered, such as its use in patients requiring surgery (to reduce a postoperative clot) and specifically studying its effect in patients with extravasation on contrast CT—indicating ongoing bleeding (the spot sign) [40, 141]. Some answers will come with STOP-IT, a prospective randomized, double-blind trial comparing rFVIIa with placebo for treatment of patients who have a spot sign on CT angiography.
Hemostatic drugs remain useful—if not essential—in rapid reversal of warfarin effect, but despite several studies showing rapid reversal of international normalized ratio, no study has definitively shown an impact on outcome [49, 93, 121, 125, 141]. The side effects of these drugs remain concerning, although a recent study found predominantly deep venous thrombosis usually in patients at risk. Despite use of rFVIIa, the mortality and morbidity of cerebral hemorrhage remain high, and the drug likely does not affect deteriorating patients at presentation.
Short Historical Note 44
Corticosteroids in Cerebral Hemorrhage
The Title of the Paper
Poungvarin N, Bhoopat W, Viriyavejakul A, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N Engl J Med. 1987;316:1229–33.

Fig. 6.9
Title page
The Paper and the Times
Because corticosteroids may reduce brain edema, these drugs have always been of interest to physicians confronted with patients who deteriorate from swelling after a stroke [107, 130]. Corticosteroids also have been used in tumors surrounded by edema and with a major clinical effect. Before computed tomography (CT) scan could differentiate between hemorrhagic and ischemic strokes in the early 1950s, corticosteroids were suggested as the immediate therapy of “apoplectic stroke.” Russek et al. reported a preliminary report in 15 patients with apoplectic stroke who were treated with a short course of “cortisone within 48 hours of presentation.” The clinical diagnosis in these patients was “cerebral thrombosis or embolism”, but there was no later pathologic confirmation. In a follow-up study with more patients, Russek et al. reported dramatic improvement in 21 of 35 patients within 24 h after cortisone therapy.
There was not only a striking amelioration of paralytic signs and symptoms but also unquestionable benefit from changes in the mental, emotional, sensory and psychomotor status. Conjugate deviation of the eyes, incoherent speech, dysarthria, and aphasia appeared to clear rapidly during cortisone therapy. The greatest effects were noted among those who were somnolent, stuporous, mentally depressed or apathic [107].
The treatment appeared almost a panacea for stroke, and they concluded enthusiastically:
…with cortisone therapy rehabilitative measures can be instituted with little difficulty after only one or two days of treatment because of the patient’s alertness of mind or euphoria. In a disease that is commonly viewed with a fatalistic philosophy and treated at best with nursing care, cortisone should prove a most valuable addition to therapy and a useful adjunct to rehabilitation.
How to understand these phenomenal results—not seen in later studies of either ischemic or hemorrhagic stroke—has remained unexplained. There are still physicians at conferences who ask the speaker if corticosteroids work in ischemic stroke.
Corticosteroids could potentially have an effect on the disrupted blood–brain barrier. Precisely how is less known, and the operative mechanism often is explained away as “stabilization of the blood–brain barrier.” Corticosteroids also may reduce cerebral interstitial edema and then reduce intracranial pressure and damage to the brainstem. Use of corticosteroids seemed random and was influenced by previous claims with very few patient studies done. However, there were two important clinical studies.
First, a 1973 study by Tellez and Bauer included 40 patients with clinical evidence of acute intracerebral hemorrhage due to hypertension, hemorrhagic infarction, or cerebral aneurysm, who were treated with dexamethasone [13, 143]. Impaired consciousness or bloody cerebrospinal fluid was needed for inclusion. There was no CT scan confirmation because in the United States, CT scans were not purchased by hospitals until after 1974. No major differences in outcome were found with 100% mortality in the comatose or stuporous group and 92% in the control group. Only seven patients survived, but better motor function was found in survivors treated with dexamethasone.
Second, a 1986 study by Norris and Hachinski performed a double-blind, controlled trial of high-dose dexamethasone (480 mg over 12 days) in 113 patients with CT-confirmed ischemic stroke [130]. Outcome between the two groups did not differ significantly in mortality or morbidity. The authors warned that widespread use of corticosteroids would expose large numbers of patients with ischemic stroke to serious hazards of corticosteroid treatment.
Additionally, previous clinical trials and cohorts of patients with cerebral hemorrhage did mention the use of corticosteroids, but the decision was left at the discretion of the neurosurgeon. No information could be extracted that could explain the incentive to use corticosteroids, but it is likely that mass effect and visible edema on CT scan prompted administration of corticosteroids. Until a study by Poungvarin and associates in Thailand, the validity of the common recommendation to use corticosteroids in intracerebral hemorrhages had never been tested in a large group of patients.
The Details of the Paper
This double blind intention-to-treat study randomized 93 patients with an intracranial hemorrhage and between the ages of 40 and 80 years into dexamethasone versus placebo groups (Fig. 6.9). The study was performed in Siriraj Hospital Medical School in Bangkok, Thailand. The clinical trial, however, prematurely ended when an interim analysis showed important adverse effects. Patients received dexamethasone 10 mg initially followed by 5 mg every 6 h for 6 days, 5 mg every 12 h for 2 days, and 5 mg for 1 day.
The main study results showed no improvement in outcome but with more complications, mostly infections and diabetic complications, in the treatment group than in the placebo group (Fig. 6.9). In clinically less severely affected patients, a benefit could be demonstrated, but only after post hoc analysis of the data. This mostly involved noncomatose patients.
The study had little neurologic details other than a Glasgow Coma Scale score and no measurement of volume of the hematoma. The number of complications in treated patients was significant—ten times higher than controls—with pneumonia, septicemia, urinary tract infection, upper gastrointestinal hemorrhages, and a pronounced diabetogenic effect in almost half of the patients treated with dexamethasone.
The Message and Acceptance
After this landmark trial, another (underpowered) study followed, and data were published as a letter to the editor. Desai and Prasad randomized 26 patients within 5 days of presentation [41] (IV dexamethasone, at a dose of 4 mg every 6 h for 12 days, followed by 4 mg every 12 h for 2 days, and 2 mg every 12 h for 2 days). There were more comatose patients in the dexamethasone-treated group, but again no benefit was found. However, adverse effects were less in this shorter treatment study compared with the Poungvarin study.
The use of corticosteroids since this study has diminished substantially, but the practices are not well known. Some neurosurgeons continue to use corticosteroids in a patient with a large hematoma or hematomas in the brainstem. Others feel there is no proven benefit. No more studies have been sponsored since this randomized study, and perhaps the effect of corticosteroids remains to be explored. A recently published Cochrane analysis found often markedly underpowered clinical trials and no benefit [47]. A recent unusual comparison of two geographically different populations from different centers found a significant difference in outcome in favor of dexamethasone treatment. In the patients treated, the dose of dexamethasone increased with larger size hemorrhages. The authors suggested a new clinical trial [169].
Some continue to argue that the short course of corticosteroids in patients with intracranial hemorrhage might benefit patients, but again there is no good experimental data to support such a claim. Most physicians would be reluctant to use high dose corticosteroids in patients who often have comorbidities, and its administration could lead to exacerbation of diabetes. A modern study using corticosteroids or methylprednisolone may be useful if it would clearly identify the neurologic condition at presentation and delineate the CT scan characteristics.
Currently, corticosteroids are avoided in most neurocritical care or neurosurgical practices, unless hemorrhage occurs in a primary brain tumor or brain metastasis. Corticosteroids also remain indicated in hemorrhages associated with inflammatory processes such as central nervous system vasculitis.
Short Historical Note 45
Surgery in Cerebral Hemorrhage
The Title of the Paper
Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral hematomas in the International Surgical Trial in Intracerebral Hemorrhage (STICH): a randomized trial. Lancet. 2005;365:387–97.
Fig. 6.10
Title page
The Paper and the Times
A cerebral hemorrhage may cause mass effect and result in clinical deterioration from compression of critically important brain tissue (i.e., thalamus) and displacement of the brainstem. Relieving this pressure will improve a depressed consciousness, and neurosurgeons and neurointensivists know too well that patients can be much better after surgery. The clot not only may have a mechanical but also a toxic effect on surrounding tissue (i.e., expression of the proinflammatory mediators) that may become irreversibly damaged if the clot is not removed in time.
Removal of a hematoma from the brain—excluding patients with penetrating or closed traumatic head injury—was not commonly entertained in the pre-computed tomography (CT) era. Opinions of neurosurgeons varied widely at the time and still do. Many would only operate in patients progressing to coma or in situations of a worsening neurologic deficit. Neurosurgeons often felt that the immediate preoperative condition of the patient was far more important than the time interval from the ictus. At the time, many neurosurgeons opined that surgery should be delayed for a week.
There was a common understanding that the direction of hematoma advancement and rapidity of clot formation determined outcome. Patients comatose early after onset had a poor outcome.
Surgery for hemorrhages in the brainstem was only considered if the hematoma extended to the fourth ventricle, causing acute hydrocephalus, and successful cases were reported since Dandy’s first successful attempt.
One prospective randomized trial by McKissock and colleagues in 1961 showed that the outcome was worse with operative treatment when compared with conservative treatment [96].
When CT scans in the mid-1970s could identify cerebral hematomas, urgent intervention became more in vogue and probably was spurred by the mere sight of a clot. After McKissock’s study, clinical trials included studies using endoscopic removal of the hemorrhage, but they showed conflicting results. Most clinical trials involved deep ganglionic hemorrhages. Ultra-early hematoma evacuation also appeared harmful, and a clinical trial testing this strategy had to be terminated because of increased number of deaths among patients operated within hours [100]. Uncertainty about the effects of urgent evacuation eventually led to a large clinical trial and now is considered a benchmark in neurosurgical treatment of cerebral hemorrhage. The trial was organized with the premise that improved surgical techniques, as well as intensive care, could result in a better outcome (Fig. 6.10).
The Details of the Paper
The trial randomized 1,033 patients from 83 centers in 27 countries to early surgery or conservative treatment. Patients were eligible if they had a minimal hematoma diameter of 2 cm and the Glasgow Coma Scale sum score was 5 or more.
Patients were only eligible if they had CT evidence of a spontaneous supratentorial hemorrhage with no evidence of aneurysm or angiographically proven arteriovenous malformation within 72 h.
The clinical trial was based on the “clinical uncertainty principle.” This principle implied that if the responsible neurosurgeon was uncertain about the benefits of either treatment—conservative versus surgical—the patient could be randomized. It is unclear from the study results how many patients were rejected for surgery, but neurosurgeons did not feel strongly about it one way or another in more than 1,000 cases. Outcome was determined by the Glasgow Outcome Scale, and other disability scales such as the Barthel Index and Rankin Scale were used. The vast majority of surviving patients were followed for at least 6 months.
Disappointedly, the clinical trial showed no benefit of early surgery versus initial conservative management in patients with a cerebral hemorrhage.
The study included an equal proportion of lobar versus basal ganglia and thalamic hematoma. Volume of the hematoma was on average 40 mL in the early surgery group versus 37 mL in the initial conservative treatment group. Multiple variables were analyzed that included the site of hematoma, volume, depth from cortical surface, deficit of speech or extremity, any thrombolytic or anticoagulant treatment, and also country of origin. The study population had a high mortality, 36% in the early surgery group versus 37% in the initial conservative treatment group. Sixty-seven percent of the early surgery group and 72% of the initial conservation management had an unfavorable Rankin Scale score. The study group had intraventricular hemorrhage (IVH) and acute hydrocephalus in 42%. When a subgroup of patients without IVH or acute hydrocephalus and only superficially (<1 cm from the cortical surface) located lobar hematomas was analyzed, a favorable outcome was found in 39% of conservative group and 49% in surgical group. STICH II (surgical trial in lobar intracerebral hemorrhage) is underway to specifically look at the effect of surgery in such hematomas.
The Message and Acceptance
The STICH trial has reduced any enthusiasm for a preemptive evacuation of deep seated hematomas and, in fact, in any supratentorial cerebral hematoma in a stable patient [114]. Deep seated hematomas do not benefit from evacuation via a traditional open craniotomy that requires dissecting through healthy brain tissue [12, 79, 171]. Some case series and one randomized study indicated that stereotactic surgery could be useful in these cases, but experience in this regard is limited [142].
The STICH study concentrated on initial management of patients who appeared neurologically stable. Deteriorating patients were excluded mostly because they underwent surgery or treatment was not considered. STICH II also is not evaluating the deteriorating patient. We need to keep in mind that trials evaluating surgery in intracerebral hemorrhage typically have not enrolled rapidly deteriorating patients (in fact, “rescue” surgery took place in more than one in four patients randomized to the medical group in STICH). Although outcome is still poor in about 75% of patients, we know that patients with clinical and radiologic signs of brainstem compression or displacement can recover well after emergency evacuation. If those patients have good potential for recovery, surgery should at least be considered.
The STICH investigators were careful to point out that—despite the results of the trial—sizable cerebellar hematomas must be evacuated to avoid obstructive hydrocephalus and brainstem compression, all of which can be fatal or result in irreversible complications. Surgery also is indicated in patients with expanding lobar hematomas and hematomas with underlying vascular anomalies or tumors [117, 119].
Surgical trials in cerebral hematomas may not necessarily impact on practice. Most neurosurgeons will make the arbitrary decision to go ahead with evacuation of the hematoma based on patient’s comorbidities, age, coagulation status, neurologic condition at presentation, among other factors. Therefore, if a clinical trial would suggest that a preemptive approach is warranted, the question will remain whether this will be feasible in clinical practice. Most neurosurgeons would want to see some degree of deterioration in order to proceed.
Short Historical Note 46
Therapeutic Hypothermia After Cardiopulmonary Resuscitation
The Title of the Paper
The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56.
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63.

Fig. 6.11
Title page

Fig. 6.12
Title page
The Paper and the Times
For many years, little could be done for patients comatose after cardiopulmonary resuscitation (CPR). Not only were earlier trials on neuroprotection unsuccessful, but also the supportive care of the patient following cardiac arrest—often with an acute myocardial infarction—was less clear and not protocolized. One of the first well-organized randomized clinical trials from the Brain Resuscitation Clinical Trial group involved a study on the efficacy of thiopental loading. Presumably, thiopental could lead to a decrease in cerebral metabolic rate or a decrease in cerebral edema. Patients with no purposeful motor response to pain after reestablished systemic circulation were randomly allocated to a thiopental loading dose of 30 mg/kg given intravenously within 10–50 min. Unfortunately, thiopental loading had no benefit on outcome, and high doses resulted in severe hypotension, with many patients thus requiring vasopressors [164].
A second landmark trial was a prehospital IV administration of nimodipine. Again, immediate treatment with calcium channel blockers did not change the neurologic prognosis in patients after out-of-hospital ventricular fibrillation, but recurrent ventricular fibrillation was significantly lower in the nimodipine-treated group [126]. Unfortunately, another trial of the Brain Resuscitation Clinical Trial group using another calcium channel blocker (IV lidoflazine) did not show significant differences in outcome. The future seemed bleak with no potential for improving the often devastating effect of anoxia and ischemia to the brain.
Induced hypothermia has been an age-old treatment for acute brain injury. However, experiments in the 1950s with induced hypothermia showed complications of such intervention, perhaps because temperatures did dip into the 28°C range [14]. A panoply of complications ranged from cardiac arrhythmias (including cardiac arrest) to coagulopathies and infections. Mild hypothermia (33–36°C) did not have these side effects; more easy control of shivering and its use in animal experiments showed still better neurologic outcomes [88, 140]. In hospitals, hypothermia mostly was used to protect the brain during cardiac surgery, but it never reached the intensive care unit (ICU) and even was abandoned from the operating room. A new serious attempt organized in two different centers would appear successful.
The Details of the Paper
Two comparatively small trials were undertaken using superficial cooling of patients for a short period of time (Fig. 6.11 and 6.12). One study coordinated from Vienna and involving nine European hospitals randomized patients into standard intensive care or a hypothermia protocol combined with fentanyl, midazolam, and pancuronium to prevent shivering. Outcome was determined by the Pittsburgh Cerebral Performance Category (a scale almost identical to the Glasgow Outcome Scale, but numbered in reverse), and the investigators decided that favorable outcome was either good recovery or patients with a moderate disability but still able to work. The effect of hypothermia was significant with 55% favorable outcome in the treated group versus 39% in the normothermic group. Mortality also reduced from 55% to 41%. The authors calculated that to prevent one unfavorable outcome, six patients would need treatment, although the confidence interval suggested potentially more patients needed to be treated (i.e., 4 to 25). The second study from Australia, conducted almost in the same time period, was smaller and involved 77 patients in four hospitals, but was not randomized and assigned according to the day of the month with odd numbers for hypothermia. This study found favorable outcome in 26% of the normothermia group and 49% in the hypothermia group. Both studies were not blinded.
The Message and Acceptance
The supportive editorial by Safar and Kochanek—both strong proponents of hypothermia—and general enthusiastic responses in the ICU community created a major surge in this treatment. Endorsement by the International Liaison Committee on Resuscitation and the American Heart Association and the Society of Critical Care Medicine followed [7, 9, 105]. There has been—perhaps surprisingly—very little objection to therapeutic hypothermia, but some problems have been identified. This involved uncertainty whether level of coma was equally distributed and early termination of both trials without power calculations [103, 104].
A recent Cochrane analysis—the authors were principal investigators of the Austrian trial—found sufficient evidence to recommend therapeutic hypothermia in comatose patients after out-of-hospital arrest and CPR for ventricular fibrillation. There has been—in general—an acceptance that short-term hypothermia could help some patients and many critical care units have protocols in place. The treatment spawned an avalanche of papers—mostly reviews with more or less clever titles (“time to chill,” “cool new treatment”). Some US states have ambulances that only bring patients after CPR to hospitals with an ICU that have hypothermia protocols.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








