(1)
Division of Critical Care Neurology, Mayo Clinic, Rochester, Minnesota, USA
Abstract
This chapter includes 15 clinical syndromes seen in acute neurologic conditions. The origin of the eponyms is discussed. These acute disorders range from comatose states to acute disorders of the spine and peripheral nervous system.
Short Historical Note 19
Minimally Conscious State
The Title of the Paper
Giacino JI, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–53.
Fig. 4.1
The title page
The Paper and the Times
It is expected that most comatose patients awaken even after a major acute injury. In the early weeks, the responsiveness and awareness varies. In some patients, there appears none, in others there is merely a hint. Following initial recovery in responsiveness, these patients may stay in the intensive care unit for treatment of systemic complications, but even if these intercurrent illnesses have come under control, a marked disability may remain.
Severe neurologic injury has been mostly dichotomized as “severe disability or persistent vegetative state.” However, rehabilitation physicians recognized that patients in a supposedly persistent vegetative state (PVS) were sometimes more awake, albeit minimally so. These patients most typically showed inconsistent responsiveness, and therefore, an attempt was made to further define this condition, to name it minimally conscious state (MCS) and to set it apart from PVS. Estimates were brought forward that claimed MCS would be ten times more common than PVS. However, it did not appear obvious to neurologists that different states of prolonged consciousness could exist, different outcomes could be expected, and that there even could be different therapeutic approaches [21].
The Details of the Paper
In 2002, the Aspen Consensus Conference Work Group suggested a condition of severe disability with minimal awareness. Nine meetings were held with delegates in bioethics, neurology, neuropsychology, neurosurgery, physiatry, nursing, and allied health (Fig. 4.1). When patients did not fulfill the criteria of a PVS, they suggested using the term minimally conscious state (MCS). However, the definition (or boundaries) of this disabled state was neither based on prospective data nor was there much known about the chances of improvement. The working group emphasized that the distinction of MCS from PVS was mostly a partial presence of awareness. In the working group’s opinion, this characteristic had medicolegal implications and questions about the use of resources, but there was no further explanation about the consequences of such a distinction.
Although patients in an MCS are aware, to recognize and demonstrate awareness is difficult if the patient does not follow a command. A simple motor task does not imply full awareness, and obviously, a gesture or verbalization is a more useful sign. To the physician observing these patients behaviors should be purposeful, although patients’ responses could be slow. In all patients, the level of awareness was markedly diminished.
A definition of MCS was proposed: “The minimally conscious state is the condition of severely altered consciousness in which minimal but definite behavioral evidence of self or environmental awareness is demonstrated.” Diagnostic criteria were proposed: “the following simple commands, gestural or verbal yes and no responses, intelligible verbalization, purposeful behavior including movement or effective behaviors that occur in contingent relation to relevant environmental stimuli and are due to reflexive activity.” The working group also suggested future directions for research to study the incidence and prevalence of MCS, natural history, and recovery course but also interrater and intrarater reliability of the diagnostic criteria.
Criteria for emergence from MCS also were proposed and included the appearance of interactive communication and functional use of two objects.
The working group acknowledged that the incidence and prevalence, the ability to be diagnosed accurately, the course of recovery, and potential for treatment remained largely unknown.
The Message and Acceptance
The American Academy of Neurology endorsed the document that was proposed by the Aspen Working Group.
Now looking back, the term minimally conscious state is most useful for patients with certain neurologic characteristics, but it likely is a mixed bag of neurologic conditions [37, 197, 198]. The transition to a better than minimal conscious state also is poorly defined, and using these aforementioned criteria may, on the surface, categorize quite a large proportion of patients with severe brain injury in this state. Those who care for patients in neurointensive care and trauma units may even question who is not in a MCS.
Moreover, a recent study found the current criteria of giving accurate answers to simple yes and no questions problematic in patients with traumatic brain injury and suggested the MCS criteria could result in misclassifying patients with cognitive disabilities [120].
Perception of pain in MCS- and perhaps suffering from it- has been very difficult to assess. One study applied a noxious stimulus (nerve conduction stimulus) to the median nerve and found activation of the primary sensory cortex, thalamus, frontoparietal and anterior cingulate cortex in MCS similarly as in controls, but much less so in PVS [31]. These findings, however, do not resolve the issue because it remains unclear how to reliably assess pain perception.
In the United States, MCS became accepted after the case of Terri Schiavo. Attorneys for the Schiavo family suggested Terri could be in a MCS. The publicity surrounding this case brought this category of abnormal consciousness into the mainstream and—incorrectly—suggested that an easy distinction between MCS and PVS was feasible clinically. Moreover, several years later, functional magnetic resonance imaging scan studies suggested that neurologic examination may not be sufficient and that these tests could actually identify patients in an MCS previously diagnosed as being in a PVS [108, 116, 166, 190]. Furthermore, studies suggested that MCS could result in further improvement and that PVS could potentially improve into an MCS. Most patients emerge from MCS in 3 months, and improvement may occur later, but more than 60% of the patients will remain severely disabled and fully dependent on others.
It is thus likely that MCS is a transitional condition leading to a severely handicapped state that requires full nursing care to maintain body integrity. Families cannot expect any participation in decision making. The emotional state, if any, may be bland and not responsive to medication. Attempts to change this deplorable situation with deep brain stimulation have resulted in some improvement in responsiveness, but whether this intervention will show promise is unclear [164, 165]. A recent study however suggested that amantadine could speed up recovery in some patients [68]
Short Historical Note 20
Persistent Vegetative State
The Title of the Paper
Jennet B, Plum F. Persistent vegetative state after brain damage: a syndrome in search of a name. Lancet. 1972;1:734–7.

Fig. 4.2
Title page
The Paper and the Times
Severely injured patients with no evidence of awareness of their surroundings and no prospects for improvement have been known for centuries. When neurology matured as a specialty, there was not only an incentive to better diagnose these states, but also to mark the dismal nature of the condition.
Over the years, there have been many attempts to find a fitting and workable term. Dejerine possibly coined the term coma vigil to distinguish it from other types of coma. The more severe types of coma were mostly called coma carus and were defined as a patient who can hardly be aroused, would only open the eyes after a strong stimulus, and could barely move. In coma vigil, none of that would exist, but the patient’s eyes were spontaneously open [56]. (Coma dépassé would become the next stage; see historical note 21). Jouvet introduced the term la stupeur hypertonique post–comateuse, but the term lacked sufficient details and was untranslatable. Other terms used by French neurologists were vie vegetative or coma prolongé. Kretschmer named it Das apallische syndrom. The term indicated an abnormality of a lesion of the pallium also known as the mantle of the gray matter forming the cortex. In 1969, in the United States, Adams and Jequier already described these patients as being in “a most primitive vegetative state” [6].
Since its original description, the term persistent vegetative state (PVS) has remained unchallenged [191]. Short of a better alternative, PVS has resonated well for physicians. Patient’s families may occasionally say “he never wanted to be kept as a vegetable” (often referring to a less severe disability), or some may use the Terri Schiavo case as an example “she never wanted to be like the Schiavo case [10].”
The Details of the Paper
Bryan Jennett—having seen a significant number of patients after traumatic brain injury—approached Fred Plum to write this paper together (Fig. 4.2). In their 1972 communication, Jennett and Plum coined the term persistent vegetative state. In their 1972 Lancet communication, they wrote:
The word vegetative itself is not obscure: vegetate is defined in the Oxford English Dictionary as “to live a merely physical life, devoid of intellectual activity or social intercourse,” and vegetative is used to describe “an organic body capable of growth and development but devoid of sensation and thought.”
Jennett used the term vegetative to emphasize the vegetative component of the nervous system. Plum noted that he could have called it “persistent autonomic state,” but felt that it was less flexible.
The major focus of this paper was to call attention of a group of patients with undeniable clinical characteristics, but Jennett and Plum understood that transitional cases could exist. “Although we would not deny that a continuum must exist between this vegetative state and some of the others described, it seems wise to make an absolute distinction between patients who do make a consistently understandable response to those around them, by word or gesture, and those who never do.”
Jennett and Plum were also quick to point out:
New methods of treatment may, by prolonging the lives of patients with conditions which were formerly fatal, result in situations never previously encountered. And new situations call for new names if they are to be accurately understood and discussed. … There is a group of patients who never show evidence of a working mind. This concept may be criticized on the grounds that observation of behavior is insufficient evidence on which to base a judgment of mental activity; it is our view that there is no reliable alternative available to the doctor at the bedside, which is where decisions have to be made.
Jennett and Plum felt that hope for clinical recovery was unrealistic. In order to increase confidence in the diagnosis, they called for more clinical studies and questioned how long PVS should persist before it can be declared permanent. This would have to be determined by prospective studies.
The Message and Acceptance
The message by Jennett and Plum was very clear—some patients are permanently unconscious, and they should be recognized as a separate category [88]. The neuropathologic correlate is often a severe diffuse cortical injury with atrophy, but there may be more pronounced abnormalities in the thalami [4].
The clinical condition was further scrutinized by a Multi-Society Task Force (see historical note 57). Although a presumptive diagnosis could be made earlier, it was suggested that a diagnostic neurologic examination for the purpose of establishing a definitive diagnosis of a PVS should be postponed until at least 1 month has passed.
A basic neurologic examination may not capture the salient features of PVS. Careful examination of the eye movements has a high priority. Eyes may open wide when patient is touched, but visual pursuit—smoothly following an object—is absent or at the very least momentary and not reproducible. Visual fixation is absent, although it can appear later and mostly at random without other signs of improvement. A visual orienting reflex may occur with head turning when family members or nursing staff move in the room. Large objects or persons suddenly closely approaching the patient may result in the patient briefly turning the eyes and suggest target focusing, but the response extinguishes quickly. The task force recognized that one should not make a diagnosis of PVS when there is any degree of sustained visual pursuit or reproducible visual fixation.
In a later paper, Jennett summarized the current state of the terminology [89]:
What attracts attention and curiosity is the dissociation between arousal and awareness—the combination of periods of wakeful eye opening with lack of any evidence of a working mind either receiving or projecting information. The advantage of the term “vegetative state” is that it simply describes observed behavior, without implying specific structural pathology.
Misdiagnosis of PVS has been reported [9, 118]. The reliability of the diagnosis of a PVS has been surveyed in the United Kingdom and in the United States, and traces of awareness were found in a considerable proportion of patients deemed to be in a PVS. Misdiagnosis was more likely in patients identified as being in a PVS within 3 months of injury and after trauma. Conversely, patients who reside in long-term care facilities may not have been diagnosed as being in a vegetative state. Indistinct terms (e.g., severe brain injury, or even comatose) may be used, and far more importantly, families may not have been informed about the permanent nature of the brain injury. The reasons are unclear, but most likely pertain to insufficient expert assessment of these patients and much less likely recovery when the patients—for some legal reason—are extensively reexamined. It also remains to be seen whether some subgroups of patients exist that may be in a condition in between PVS and MCS (MCS plus or PVS minus.)
For most expert neurologists, the diagnosis of PVS is quite simple and straightforward, recognizing that multiple examinations over time would be most accurate. For non-neurologists, the diagnosis can be challenging and not infrequently nursing staff or families may notice more. Ignoring their call is likely a major factor in misdiagnosis.
A European task force on disorders of consciousness suggested to rename the condition “unresponsive wakefulness syndrome.” The reason was the alleged “negative connotation” and “pejorative undertone”; a concern so often put forward by pro-life groups. The working group also felt that once using the term PVS, the signs of recovery of consciousness would be missed. Whether changing the term to unresponsive wakefulness syndrome would prevent that from happening is unknown and unlikely [100].
Sadly, there is still a misunderstanding among some physicians and health care workers about the meaning of the terms brain death and vegetative state. In fact, most catastrophic injury spares the brainstem and does not lead to PVS or brain death. Both conditions represent the extremes of neurologic injury.
Short Historical Note 21
Brain Death
The Title of the Paper
Mollaret P, Goulon M. Le Coma Dépassé (memoire preliminaire). Rev Neurol. 1959;101:3–15.

Fig. 4.3
Title page
The Paper and the Times
Death of the brain—meaning death of the person—was quite a departure from the traditional clinical diagnosis of death that required irreversible absent breathing and circulatory arrest. After a catastrophic neurologic injury, patients would follow a common pathway, become comatose, and develop respiratory failure leading to insufficient breathing, apnea, and circulatory arrest. The mechanical ventilator would change that course. Now, patients were placed on a ventilator and the brain injury could progress to involve the disappearance of all brainstem reflexes. Even before criteria were published, most physicians felt that these patients were essentially dead.
These clinical observations became more pertinent when electroencephalographers found that the electroencephalogram (EEG) showed no activity and neuropathologists found complete necrosis of the brain.
Although most initial observations pertained to EEG findings, Lofstedt and von Reis reported in 1956 six mechanically ventilated patients with absent reflexes, apnea, hypotension, hypothermia, and polyuria. Cerebral blood flow, determined by angiography, was absent [107]. Cerebral necrosis was present at autopsy in all cases.
In 1959, Wertheimer, Jouvet, and Descotes were among the first to propose criteria for this new clinical state [193]. This manuscript largely focused, as many before, on the significance of an isoelectric EEG but also documented shutting off the ventilator to stimulate the respiratory centers with increasing respiratory acidosis. Absent medulla oblongata function was further confirmed with no change in pulse rate with carotid compression, ocular pressure, and IV injection of atropine and amphetamine.
Both these papers, although introducing new findings lacked neurologic details that would come with the work by Mollaret and Goulon (Fig. 4.3). Almost 10 years would pass until the issue was comprehensively dealt with by a committee of physicians and other stakeholder specialties from Harvard Medical School.
The Details of the Paper
The paper involves 23 cases at Claude Bernard Hospital in Paris that were called Coma dépassé. The authors described in sufficient detail absent brainstem reflexes, apnea, and also polyuria and difficulty maintaining blood pressure with vasopressors. A flat EEG also was noted by the authors. Oxygen desaturation and respiratory metabolic acidosis were commonly observed, and intramuscular injection of vasopressor was needed to maintain a systemic hemodynamic stability. Coma dépassé was said to be characterized by immobility of the eyeballs in a neutral position, mydriasis, absent light reflex, absent blinking with stimuli, absence of swallowing reflexes, drooping of the jaw, absence of motor responses to any stimuli, muscle hypotonia, tendon areflexia, equivocal plantar reflexes, absence of spontaneous respiration after discontinuation of ventilation, immediate cardiovascular collapse as soon as vasopressors are stopped, and a disturbance of thermoregulation with core temperature, depending on the environmental temperature. The authors compared this clinical state with other types of coma and pointed out differences (Fig. 4.4).
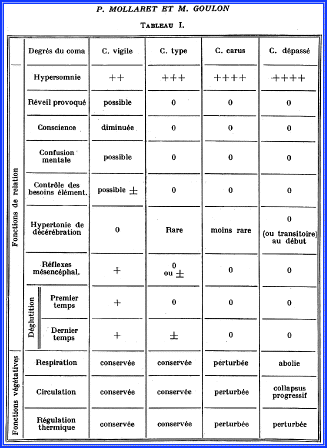

Fig. 4.4
Comparison with other types of coma (most notable difference with coma carus [deep coma]) is respiration pertubeé (abnormal breathing) to respiration abolie (absent breathing) and circulation collapsus progressif (progressive circulatory collapse)
Mollaret and Goulon found cases in which mechanical ventilation was controlled in the first days, only to deteriorate later, and they documented oxygen desaturation, acute hypercapnia, and combined respiratory and metabolic acidosis. Polyuria was present in most cases, and intramuscular injection of d’hormone post–hypophysaire resulted in reduced diuresis and concentration of urine. Hyperglycemia and glycosuria were observed, but there was a normal response to insulin. The heart rate slowed to 40–60 beats/min and was not changed by pressure on the eyeballs, carotid sinus, or by IV injection of atropine. This condition ended ultimately with cardiac arrest.
The Message and Acceptance
There has been some historical discussion as to whether neurologists Mollaret and Goulon or neurosurgeon Wertheimer and neurologist Jouvet were the first to describe brain death. Both papers—published the same year and even months in between—have important comparable attributes. A strong argument can be made that Mollaret and Goulon had a more detailed paper, larger series of patients, significant more detail on how the patient’s absence of brain function impacted on systemic instability, and further characterization of laboratory abnormalities.
Jouvet and Wertheimer were set on defining brain death by electrophysiologic criteria. They described patients as “heart-lung preparations.” The authors felt objective criteria were needed and inserted fine bipolar electrodes into the medial thalamic structures, applied strong electrical currents, and found no motor response.
After these observations, very few papers were published between 1959 and 1968. Studies largely concentrated on isoelectric EEGs and findings of absent intracranial flow. In the United States, Schwab is credited for providing a more detailed description of an isoelectric EEG in brain death and merging the EEG into diagnostic criteria [168]. These criteria would allow the physician to indicate that the patient was dead:
Absence of spontaneous respiration for 30 min
No tendon reflexes of any type
No pupillary reflexes
Absence of oculocardiac reflex (eyeball pressure slowing heart rate)
Thirty minutes of isoelectric EEG
These early observations in 1963 were a prelude to a more comprehensive definition of brain death. This focused effort would appear in 1968. In Boston, the Harvard Medical School Ad Hoc Committee to Examine the Definition of Brain Death [1] set out to “define irreversible coma as a new criterion for death” [1, 196]. The criteria of brain death were based on the collective experience of the committee members. The Harvard criteria were published in the Journal of the American Medical Association (JAMA) on August 5, 1968 (Fig. 4.5). The document, a collection of brief statements included a definition of brain death, a legal commentary, and an explanation of an address by his holiness Pope Pius XII.

Fig. 4.5
Title page
In the next development, which turned out to be crucial in the way brain death was diagnosed, Mohandas and Chou published the Minnesota Code of Brain Death [114], also known as the Minnesota criteria. Major changes from the Harvard criteria included definition of time of apnea (4 min of disconnection), exclusion of metabolic derangements, and shorter observation time of 12 h, but the paper did not present validating data. Mohandas and Chou should be credited for first introducing into the brain death literature the information that damage of the brainstem was a critical component of severe brain damage.
The centrality of the brainstem had been recognized by many. During this time, a confusing discussion appeared with new terminology. Brain death was now described by some bioethicists as whole brain death (hemispheres and brainstem irreversibly damaged) but also as higher brain death (hemispheres irreversibly damaged), brainstem death (brainstem irreversibly damaged), and even a super locked-in syndrome (brainstem irreversibly damaged but hemispheres functioning), dividing the death of the brain into segments. This discussion largely was helpful in distinguishing persistent vegetative state (higher brain death) from brain death (whole brain death), but such a categorization would ignore the typical course of massive hemispheric damage that would eventually, in a rostrocaudal way, damage the brainstem.
These discussions, culminated in a report of the Medical Consultants on the Diagnosis of Death to the President’ Commission on Ethical Issues in Medicine and Biomedical and Behavioral Research and were published in 1981 [8]. The report led to the Uniform Determination of Death Act.
The American Academy of Neurology published its guidelines in 1995 and 2010 and no other society guidelines have been published since [203].
The British neurologists understood as one of the first that the “brainstem death is the infratentorial repercussion of supratentorial events [51].” In a series of papers, Pallis described the diagnosis of brainstem death, the pitfalls and preconditions eventually bundled in the ABC of brainstem death [132–134] (Fig. 4.6).


Fig. 4.6
Pallis’ opening paper arguing brainstem death in a series of articles published in BMJ in 1982
These preconditions were essential in the approach to the patient.“Testing for brainstem death should not be undertaken unless the cause of coma has been established beyond all doubt.” Pallis clearly emphasized the importance of an apnea test and using blood gas determination. Pallis warned against apnea testing by only looking at overriding the ventilator for 15 min. “The test for apnea without ensuring and documenting an appropriate rise in PaCO2 has been likened to testing the pupils without a battery in the torch.”
Cardiac arrest is inevitable when all brainstem functions are lost. Pallis noted in 1990:
To date not a single case seems to have been recorded—in a reputable and widely accessible journal- of a patient with well documented structural brain damage (from trauma or intracranial hemorrhage, for instance) who recovered brainstem function after fulfilling properly applied clinical criteria of brain stem death [134].
Short Historical Note 22
Locked-in Syndrome
The Title of the Paper
Nordgren RE, Markesbery WR, Fukuda K, Reeves AG. Seven cases of cerebromedullospinal disconnection: the “locked-in” syndrome. Neurology. 1971;21:1140–8.

Fig. 4.7
First mention of a locked-in syndrome in Plum and Posner’s monograph on the diagnosis of stupor and coma

Fig. 4.8
Title page
The Paper and the Times
One of the most existential fears one could have is being trapped in one’s own body and being misdiagnosed as comatose. In 1844, Alexandre Dumas described such a state in the fictional character of Monsieur Noirtier, who was in this condition for more than 6 years and was described as a “corpse with living eyes.” Later descriptions of a locked-in syndrome in a novel included one by Emile Zolá in Therese Raquin, in which Madame Raquin had a stroke. The features of the syndrome were translated by Pearce as “her tongue turned to stone,” “she was struck dumb and motionless,” “she only had the language of her eyes,” and “she could communicate quite easily with an imprisoned mind buried alive in a dead body” [137].
The medical literature, however, lacked a clinicopathologic correlation and a detailed description of the eye movement abnormalities. Being paralyzed and unable to communicate while wide awake was perhaps best known from using neuromuscular drugs during anesthesia. Decamethonium, gallamine, and suxamethonium were discovered in the early 1950s. Neurologic causes for being “locked-in” were often intoxications. For example, Davis et al. described a case resembling locked-in syndrome caused by diazepam toxicity, which caused “prolonged semi-coma followed by transient muteness and tetraplegia in a patient with tetanus [53].” Most noteworthy was buckthorn neuropathy with a rapid paralysis and bulbar involvement. A brainstem location for locked-in-like syndrome had been noted by Adams and Victor in central pontine myelinolysis.
The term locked–in syndrome was based on a single case described in detail in the first edition of Plum and Posner’s monograph on stupor and coma (Fig. 4.7). The 44-year-old man described had a basilar artery thrombosis. He was examined 1 month after the ictus. He was awake and appropriately moved his eyes up and down on commands and to indicate yes or no. There was no “lateral motion of the eyes either on command or on passive head turning.” In addition, “there was no voluntary movement in structures innervated by cranial nerves IX–XII.” The electroencephalogram (EEG) was normal. The patient developed some motion in the ankle, but died from pneumonia. Autopsy confirmed the diagnosis with severe atherosclerosis of the basilar artery, a cystic infarct in the upper two-thirds of the base of the pons and an infarction of the cerebellar peduncle. Autopsy also revealed that the reticular formation was not involved. One of the first mentions after this case report was by Chase et al. [48] and most notably by Nordgren, who summarizes the literature adding four cases (Fig. 4.8).
The Details of the Paper
Nordgren and associates saw an extraordinary number of cases in a short time period. Three cases were due to basilar artery thrombosis, and one was a hemorrhage into the fourth ventricle. A careful clinicopathologic correlation was described with illustrations showing the extent of involvement in some cases. The authors considered it a variant of akinetic mutism, but were quick to point out the major differences in neurologic examination and site of the lesion. Consciousness was preserved, but patients had decerebrate posturing after a noxious stimulus was applied.
In one case with bilateral involvement of the pontine tegmentum, the patient remained alert, suggesting that the reticular formation rostral to the pons was preserved. Further extension into the rostral tegmental levels resulted in loss of consciousness, and extension into the medulla oblongata resulted in fatality.
Most patients had an ataxic breathing defined as “unpatterned in amplitude, rhythm and rate.” Apneustic breathing with deep inspiratory holding—previously associated with pontine tegmentum lesions—was not found. Others had deep and rapid respirations as seen in central neurogenic hyperventilation.
The authors warned, “many presumed comatose patients could well be in this alert but helpless state. Unless commanded to elevate or depress their eyes, alertness would go unrecognized and they remain truly locked in.”
The authors also elaborated on some of the ethical issues associated with being in a locked-in syndrome:
While most patients with the lesions described herein will extend their infarction or hemorrhage and not survive, Case 1 of this series raises moral and ethical considerations that can be distressing for the personnel and family involved. He survived with a minimum of support for many months in an alert but completely helpless state. He was indeed completely locked in, except for his eye movements, and appeared to have no intellectual deterioration in spite of his profound motor deficit. It is worth emphasizing that considerable effort should be made to enrich the environment of these unfortunate individuals by whatever means are available to the auditory and visual spheres.
The Message and Acceptance
The term locked–in syndrome was introduced by Plum and Posner in 1966 but other terms, such as pontopseudocoma, ventral pontine syndrome, de–efferented state, pontine disconnection syndrome, and Montecristo syndrome continue to surface. One of the first citations of locked-in syndrome was made 2 years later by Shafey et al. who called it the ventral pontine syndrome [171]. In 1974, Hawkes et al. confirmed a normal EEG in a patient with locked-in syndrome. His seven patients showed an α- or τ-rhythm that reacted to noise, painful, or photic stimuli [75, 76].
The term has been overused. It has been confused with persistent vegetative state and minimally conscious state. The crucial distinction of the presence of a disconnect between horizontal and vertical eye movements in an alert patient with a paralysis of all motor function below the mesencephalon should be appreciated. Locked-in syndrome may not be recognized in patients who appear motionless.
The pathologic lesion in locked-in syndrome interrupts the corticobulbar and corticospinal tracts, but the tegmentum of the pons and midbrain, which harbors the reticular formation, is preserved, and thus, consciousness is fully retained. In patients with a basilar artery occlusion, extension of the infarct into the tegmentum and involvement of the thalamus, however, may further decrease the level of consciousness.
Voluntary eye movements in the vertical plane and blinking remain because the oculomotor and trochlear nuclei and the center for vertical gaze remain intact. A significant skew deviation may occur along with ocular bobbing.
The most recent dramatic and sad description of locked-in syndrome is that by Jean-Dominique Bauby, editor in chief of the Parisian magazine Elle, who had a devastating stroke in the brainstem. His book is titled, The Diving Bell and the Butterfly, referring to the heavy corporeal trap—the “Diving Bell”—and his imagination wandering off in space or time—the lightness of a “Butterfly.” Bauby often strove to savor the pleasures that were left to him, such as smells and vision. With a feat of great willpower and a devoted speech therapist, he blinked at the letter he wanted to produce a 137-page book describing his ordeal before he died [14]. The book provides useful insight into the terror of this condition.
In most patients, the locked-in state remains unchanged, but incomplete states have a much higher proclivity of major improvement. In complete states, some improvement may occur, such as movement of fingers that allows better signaling. However, recovery from locked-in syndrome has been reported in patients with herpes simplex encephalitis, after a bout of basilar artery migraine, multiple sclerosis, and following uncal herniation from a lobar hematoma [24, 26, 36, 177, 200]. In most of these cases, improvement occurred within days to weeks after presentation. Late recovery has not been reported, but patients may remain cognitively intact for years [43, 125, 126, 180]. A large proportion of patients die from pulmonary complications, but some patients have survived for many years, including one patient who survived for up to 27 years [72, 135].
Short Historical Note 23
Anoxic Encephalopathy After Cardiopulmonary Resuscitation
The Title of the Paper
Howkins J, McLaughlin CR, Daniel T. Neuronal damage from temporary cardiac arrest. Lancet. 1946; 1:488–92.
Bell JA, Hodgson HJF. Coma after cardiac arrest. Brain. 1974;97:361–72.

Fig. 4.9
Title page
Fig. 4.10
Title Page
The Paper and the Times
Resuscitation after cardiac standstill likely started in the operating room and in extreme circumstances. Intraoperative massage after cardiac arrest had been used for many decades. How it would affect the brain and how it would present itself was less well known. Very few neurologists concerned themselves with postsurgical disasters and descriptions were done by non-neurologist clinicians. Most initial observations came from surgeons who experienced intraoperative cardiac arrest during surgery and were left with a patient who never awakened. Perhaps one of the first cases describing clinically injured brain after successful resuscitation is by Mollison, an ear, nose, and throat surgeon from Guy’s Hospital in England in 1917 [115]:
A 6-year-old boy developed shock was flaccid, stopped breathing with absent corneal and pupil reflexes. After abdominal opening the right hand was inserted between the liver and diaphragm and the left hand on the chest wall and fingers of the right hand behind the heart. Pressure was exerted at about the rate of ninety times a minute. … Some respiration movement began attributed to blood driven to the medulla and stimulating the respiratory center but no heart contractions occurred. One cc of pitruitin was injected into the heart resulting in “strong heart beating. … For seven days the boy was more or less unconscious…for ten days there was rigidity of the limbs or choreic movements. At one time both feet and hands were held in the position of tetany. … For thirty-six hours the screaming was almost continuous … [and] has symptoms of severe cerebral irritation, no doubt due to the damage done to the brain during cessation of the circulation. …He made eventually a perfect recovery.
With the introduction of cardiopulmonary resuscitation (CPR), cerebral damage became recognized by clinicians and first by Negovsky who named it postresuscitation disease [121]. Some of the early explanations included cerebral reoxygenation injury or postischemic vasospasm leading to a necrotic process.
The devastating consequences to the brain became gradually known through careful examinations. When the more severe cases came to autopsy, neuropathologists would notice congestion of the gray matter and poor demarcation of the gray/white matter areas. Under the microscope, necrotic zones were seen in the cortical laminae, with bands of cellular vacuolization [7, 103, 117, 167].
Some of the attention was directed to the different vulnerability of gray and white matter, and it became clear that the cerebral and cerebellar cortices were more affected from ischemia than the basal ganglia—with the reverse in hypoxemia. Animal experiments also showed that white matter was more resistant to anoxic–ischemic injury than gray matter [103].
Overtime—only after carefully examining patients in persistent coma—the clinical descriptions and methods of prognostication became more sophisticated, but had to wait until the 1980s.
The Details of the Paper
Howkins paper on neuronal damage from cardiac arrest can be considered one of the first comprehensive papers on the evaluation of a patient who had successful cardiac massage (Fig. 4.9). The report is before CPR became common place. The patient is a 32-year-old woman who—after surgery was almost completed—had a sudden cardiac failure and transdiaphragmatic cardiac massage was started and lasted 7 min. She also received intracardiac adrenaline. Six hours later, her neurologic examination showed absent corneal reflexes, increased knee jerk, clonus, extensor plantar responses, and significant decerebrate rigidity. Lumbar puncture was performed and showed normal pressure. She remained comatose. The case report describes a rather complete neurologic examination with examination of the cranial nerves, motor function, tone, and reflex pattern. The EEG report showed very low voltage waves. Additionally, at autopsy, many of the anterior horn cells were shrunken and stained darkly on the lumbar cord. The midbrain showed little abnormality, and the medulla oblongata and pons also were largely spared with a few shrunken cells. The Purkinje cells in the cerebellum were reduced in number with many empty baskets. The basal ganglia showed extensive and severe changes, particularly in the caudate nucleus and putamen. Great loss of pyramidal cells was also found in the cornu ammonis.
Another work that has been often cited and was one of the largest series of coma after cardiac arrest is by Bell and Hodgson [17] (Fig. 4.10). This series shows the outcome of patients studied in the Saint Thomas Hospital: 284 patients resuscitated from cardiac arrest with 133 patients comatose. For the first time, this paper analyzes the poor prognosis of coma after cardiac arrest with only 19% of comatose patients living to be discharged from the hospital compared with 54% of noncomatose patients.
The Message and Acceptance
These reports introduce the neurology of prolonged coma after cardiac arrest and in some the evolution of a persistent vegetative state. The authors made the observation that cortical neurons die after 5 min of complete arrest.
These observations became even more important when the clinical landscape changed with the introduction of CPR standards set by the American Heart Association in 1966 [45]. Clinicians now noted that despite successful intervention, the brain still could have irreparable damage.
A major change came when Peter Safar from the University of Pittsburgh introduced “brain-oriented intensive care for coma” also known as “cerebral resuscitation [158–160].” An ambitious plan was created that included corticosteroids, temperature control, osmotic agents, and the use of barbiturates. Unfortunately, clinical trials with possible neuroprotective drugs were unsuccessful, and none of the interventions proved to be successful. A breakthrough came with the observation that early induced hypothermia to 33°C could improve mortality and morbidity [81].
As recently as 1970, there was little known on prognostication of neurologic injury after cardiac arrest. By that time, absent brainstem reflexes were clearly identified as indicative of poor outcome, and over the years, a more detailed definition of brain death emerged. However, most patients after cardiac arrest did not lose all brainstem reflexes but remained comatose with normal pupil, corneal, and oculocephalic reflexes.
How the neurologic examination could predict outcome remained elusive, and the leading neurology textbooks did not address the topic. The general impression was that duration of anoxia to the brain, duration of coma, and EEG were indicators of poor outcome [47, 172]. Studies correlating EEG with clinical findings often found in retrospect that coma lasting less than 2 days resulted in good outcome. A major study from Royal Adelaide Hospital in New Zealand reported by neurologist John Willoughby, who examined all 48 cases personally in detail, found that patients who did not respond purposefully 1 h after cardiac arrest had a 56% mortality and high likelihood of “intellectual impairment [205]”. A prospective series focused on prognosticating morbidity, and mortality was published by Levy and associates (see Historical Note 53 [105]).
Short Historical Note 24
Perimesencephalic Hemorrhage
The Title of the Paper
van Gijn J, van Dongen KJ, Vermeulen M, Hijdra A. Perimesencephalic hemorrhage: a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology. 1985;35:493–7.
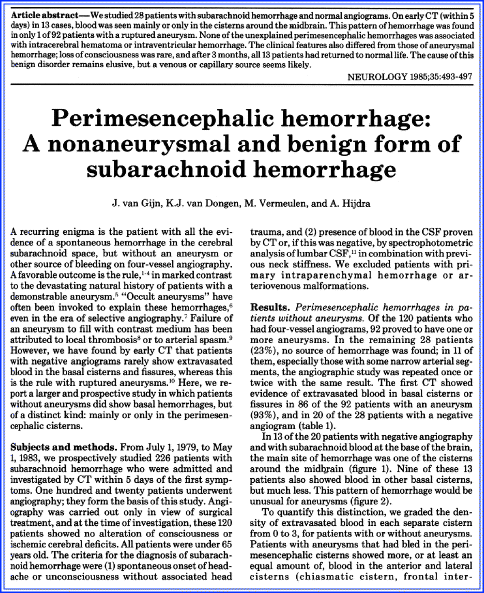
Fig. 4.11
Title page
The Paper and the Times
Aneurysmal subarachnoid hemorrhage in the early 1980s had been well characterized clinically, not only its initial presentation but also its complications. In this time period, most patients with aneurysmal subarachnoid hemorrhage (SAH) were operated on usually after the period of cerebral vasospasm had subsided and often at the end of the second week from presentation.
Most patients with an aneurysmal SAH had a cerebral angiogram except those presenting in a very poor condition or the very old, When the first cerebral angiogram showed no aneurysm, a second and even a third cerebral angiogram was performed to reliably exclude a ruptured aneurysm. The yield of these repeated studies was initially relatively high with up to 20% of cases showing an aneurysm on the second cerebral angiogram. For neurosurgeons, some concern remained even when patients had negative cerebral angiograms. Repeatedly, studies appeared that emphasized a much better outcome when no aneurysm could be demonstrated. The assumption was that the aneurysm had destroyed itself during rupture and the artery had healed naturally. Rebleed rates in these patients were very low.
After a retrospective review of all computed tomography (CT) scans of patients with such a negative angiogram—conducted in Rotterdam, Erasmus University Hospital (Dijkzigt)—a pattern became apparent that had not been noted before. Patients with a normal cerebral angiogram but demonstrable SAH on CT scan had blood mainly or only in the cisterns around the midbrain [185]. This prompted the term perimesencephalic subarachnoid hemorrhage.
This finding led to a fundamentally new insight in the evaluation of a patient with a SAH and had great consequences for treatment and follow-up of the patients.
The Details of the Paper
The initial paper involved 28 patients with SAH and a normal cerebral angiogram. In 13 patients, blood was largely located in front of the brainstem, ambient cistern, and quadrigeminal cistern. This pattern was different from an aneurysmal pattern where blood was distributed throughout the cisterns and fissures (Fig. 4.12). When the clinical features of nonaneurysmal perimesencephalic hemorrhages were compared with patients who did have more aneurysmal patterns, striking differences were noted. Headache was more gradual in the perimesencephalic hemorrhage group, and outcome was invariably good with no rebleeding or cerebral infarction, although acute hydrocephalus was found with one patient requiring a ventriculostomy. The authors speculated that this type of hemorrhage was not an arterial hemorrhage, but more likely venous. This was also considered plausible because several patients had a sudden head turn at the onset of headache, or headache started after the patient overreached—all tentatively suggesting friction of a cerebellar vein against the tentorial margin.

Fig. 4.12
Computed tomography scans showing perimesencephalic hemorrhage patterns
The Message and Acceptance
A major study followed that further refined the disorder in a larger series of patients [146–149]. According to van Gijn, acceptance of this variant started with the publication of a larger paper in The Lancet, and citations increased exponentially over the years [147, 184].
The main premise has remained unchallenged. Patients invariably presented in a good clinical condition and were rarely very much affected by it. Once a cerebral angiogram had excluded a posterior circulation aneurysm, rebleeding did not occur, cerebral vasospasm did not lead to cerebral infarction, and the outcome was exceptionally good.
Later, magnetic resonance imaging found that the hemorrhage was not so much in the perimesencephalic region, but largely in front of the entire brainstem [163]. Over the years, multiple observations have claimed to have found the cause of a nonaneurysmal perimesencephalic hemorrhage.
Candidate vessels for rupture into the prepontine and interpeduncular cistern are the basilar artery, anterior pontomesencephalic vein, superior cerebellar artery, and any of the perforating arteries in the vicinity [80, 201, 202].
Intramural dissections may have been the culprit. Arguments for this explanation included: (1) small hemorrhage would fit with a dissection, (2) common wall irregularity, often misinterpreted as early vasospasm, and (3) presence of infarcts in the pons in some cases [163].
More recently, a three-dimensional cerebral angiogram in a patient showed that small basilar artery outpouches may appear and then disappear. Thrombosis and recanalization may explain the radiologic findings of reappearance of a small aneurysm after follow-up angiogram [194].
Other studies have repeatedly shown abnormal venous patterns in patients with perimesencephalic nonaneurysmal hemorrhage, but in patients examined, venous structures in the interpeduncular or prepontine cisterns are without any abnormalities except for an occasional engorged anterior pontomesencephalic vein. No patient with a nonaneurysmal perimesencephalic hemorrhage has come to autopsy soon after the initial hemorrhage.
The discovery of a separate entity in a population of patients with “angiographic-negative subarachnoid hemorrhage” meant that neurologists and neurosurgeons now had to specifically distinguish between three CT patterns: “perimesencephalic,” “aneurysmal,” and “nonaneurysmal – nonperimesencephalic.” Each of these CT scan patterns could indicate a certain vascular lesion on cerebral angiogram or could indicate there was none. Outcome would be much different. The CT pattern has now been recognized in most medical centers throughout the world.
Short Historical Note 25
Basilar Artery Occlusion
The Title of the Paper
Kubik CS, Adams RD. Occlusion of the basilar artery: a clinical and pathological study. Brain. 1946;69:73–121.
Caplan LR. “Top of the basilar” syndrome. Neurology. 1980;30:72–9.

Fig. 4.13
Title page
Fig. 4.14
Title page
The Paper and the Times
Ronnov-Jensen claims the very first description of a basilar artery occlusion may have been in Dumas’ Three Musketeers where he describes symptoms that could have been a traumatic vertebral dissection [154]. Pathologists were aware of an acute embolus to basilar artery as early as 1900s. The first description is most likely by the Scottish physician John Abercrombie in his book, Pathological and Practical Researches on Disease of the Brain and the Spinal Cord. This observation was recently summarized in a historical article [96]. The paper describes an 18-year-old man who had a left hemiplegia followed 1 month later by loss of speech and dysphagia, and at autopsy a blocked basilar artery filled by “a firm white matter without any appearance of blood.” The diagnosis was considered “plugging of the basilar.”
Other incidental cases have been described by several physicians, and the contributions of French neurologists—Charles Foix in particular—have been recognized, leading to multiple eponyms associated with brainstem syndromes [41, 64, 65, 169]. Foix gained an eponyrm after describing a vascular syndrome in the brainstem involving the red nucleus, but he became better known by the Foix-Alajounine syndrome of the spinal cord.
According to Raymond Adams, the most completely examined case of basilar artery thrombosis was in 1932 by Pines and Gilinsky and showed a serial section of the brainstem and clear delineation of the infarctions [141]. At the time, the diagnosis or warning signs were seldom made while the patient was still alive, and autopsy often revealed surprising findings [86]. Denny-Brown may have introduced the term vertebrobasilar insufficiency, which gradually has been replaced by the term vertebrobasilar transient ischemic attacks [57].
The Details of the Paper
Kubik and Adams’ paper summarizes 18 carefully examined cases with autopsy (Fig. 4.13). The paper summarized multiple areas of occlusion with concomitant clinical features. The paper by Kubik and Adams was important because it documented for the first time that the clinical syndrome of progressive brainstem dysfunction could be associated with thrombosis of the basilar artery. The mortality was as high as 90%. The paper described single cases that presented with progressive clinical signs that included tinnitus and vertigo followed by swallowing difficulties and pupillary abnormalities and gradually lapsing into coma:
The onset is abrupt, frequently with loss of consciousness and with signs indicating a lesion in the mid-brain and/or pons. In the majority of cases there was evidence of bilateral involvement. Outstanding features are various combinations of (1) pupillary disturbances: (2) ocular palsies and other cranial nerve palsies; (3) dysarthria: (4) bilateral extensor plantar reflexes; (5) hemiplegia, quadriplegia: (6) commonly a marked remission of symptoms which is usually temporary but may occasionally end in recovery.
The paper focused on a clinical pathologic correlation. Many patients had thrombi formed on severe atherosclerotic vertebrobasilar arteries. Cardiac emboli were considered another possibility in 7 of 18 patients, but the embolus was rarely seen and probably had lysed.
In the past, cerebral vasospasm often was put forward as a cause of this syndrome, but Adams completely discounted that as a possibility. They concluded that the entity could be recognized and that patients could recover. “It should be possible in most cases of basilar artery occlusion to make a correct diagnosis. The disease is not always fatal. Histories are submitted of four patients who have recovered.”
Another major work was by Louis Caplan, who suggested a clinical diagnosis of an embolus lodged at the top of the basilar artery (Fig. 4.14). These patients presented with a rostral brainstem infarction. The concept here was that an embolus that could traverse a smaller vertebral artery would not necessarily occlude the basilar artery, except more distally where the basilar artery was tapered. Atherosclerosis of the vertebral basilar system was widespread in the reported patients.
The syndrome was characterized by visual field defects, disorders of vertical gaze, convergence, a skew deviation, and pupillary abnormalities mostly resulting in small and poorly reactive pupils. Behavior abnormalities that included hallucinosis were common, and visual field defects including visual perseverations and scintillations in an optic field were described. Many of the infarcts were bilateral and a cortical blindness or a Balint’s syndrome was noted. Patients with thalamic lesions could not form new memories and could be permanent in patients with paramedian thalamic infarcts.
The Message and Acceptance
Initially smaller brainstem infarcts were described in which patients have a constellation of findings that could localize to a specific lesion in the pons, medulla, or even mesencephalon. In some patients, rapid infarction involving the pons, thalami, and the occipital lobes could be associated with occlusion of the basilar artery or the more distal portions. Both papers from Harvard Medical School clearly defined syndromes of acute occlusion of the basilar artery with rapid progression.
Raymond Adams studied 1,400 brains at the Mallory Institute of Pathology. According to his biographer Laureno, this detailed manuscript brought Raymond Adams international recognition [99]. The “top of the basilar” syndrome also has become a medical classic and has been recognized as a devastating stroke. One of Caplan’s major achievements has been the establishment of the New England Medical Center posterior circulation registry that allowed more detailed study of patients over time or in Miller Fisher’s words “stroke by stroke” [42, 189].
A basilar artery thrombus most permanently infarcts the pons and is associated with either prolonged coma or a locked-in syndrome, both with major clinical consequences. Early recognition of acute basilar artery embolus remains problematic and clinical signs often are not appreciated by non-neurologists.
Patients may present with difficulty clearing secretions from acute dysphagia and level of consciousness may fluctuate initially resulting in respiratory problems. Not uncommonly, the patient arrives intubated in the emergency department. Computed tomography (CT) scan often is initially normal, and a hyperdensity in the basilar artery representing clot also is often not recognized in an acute hectic setting [97]. CT angiogram is now the diagnostic test of choice, or the patient goes straight to a cerebral angiography. Early endovascular therapy in basilar artery occlusive disease has been a breakthrough intervention and there is a high likelihood of improving clinical signs with recanalization.
Short Historical Note 26
Delayed Traumatic Cerebral Hematoma
The Title of the Paper
Baratham G, Dennyson WG. Delayed traumatic intracerebral hemorrhage. J Neurol Neurosurg Psychiatry. 1972;35:698–706.

Fig. 4.15
The title page
The Paper and the Times
Nonpenetrating traumatic brain injury could produce rapid injury to brain tissue, resulting in diffuse swelling or hemorrhagic contusions. For many decades, it was known that a so-called lucid interval existed. Perfectly fine patients with no overt injury after a major accident were looked at with trepidation by neurosurgeons who knew they could get worse and dramatically quick. In the majority of cases, expanding subdural or epidural hematoma could be implicated [52].
In 1891, Bollinger described for the first time delayed intracerebral hemorrhage (ICH) after trauma and named it traumatische spät–apoplexie [28]. However, neuropathology was poorly defined, and the term included different causes of traumatic brain injury. Bollinger pointed out that trauma could cause progressive cerebral infarction followed by hemorrhage. Others attributed it to “vasoparalysis” [63] or to compression of a surface collection causing hemorrhage.
The Details of the Paper
Experience from the Surgical Neurology University of Edinburgh revealed 21 patients (from 7,866 head injuries) with delayed hemorrhages (Fig. 4.15). All 21 patients had a trauma, transient period of recovery (“asymptomatic interval”), followed by secondary deterioration. The age ranged from 5 to 83 years. The interval was 90 min to 1 day. None had neurologic deficits in the asymptomatic interval. The hematoma location is shown in Fig. 4.16.
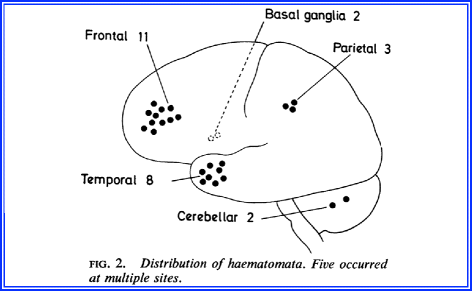

Fig. 4.16
Location of delayed cerebral hematomas
In 6 of 21 patients, there was rapid deterioration and coma before surgery. Ten of 21 patients had skull fractures. Most hematomas were in the frontal lobe. All patients required surgical treatment. The authors speculated the following:
A combination of factors, therefore, operates in the production of the delayed variety of traumatic intracerebral hemorrhage. Focal trauma produce increased flow within and around traumatized brain. Hypoxia, carbon dioxide retention, and any cause for elevation of venous pressure will increase this tendency, thus producing ideal conditions for the gradual development of an intracerebral hematoma.
The Message and Acceptance
Years later, after the use of computed tomography (CT) scan became more widespread, delayed traumatic cerebral hematoma was more easily recognized [79, 122, 124, 174]. In Soloniuk’s study, ICH was immediate (<3 h) in 20% within 3–6 h in 6% delayed (6–24 h) in 29% and much delayed (>24 h) in 46%. None of the patients had intracerebral hematoma on initial CT scan. Mortality despite surgery was 50% [173].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








