(1)
Division of Critical Care Neurology, Mayo Clinic, Rochester, Minnesota, USA
Abstract
The tools of prognostication in acute neurologic conditions have developed over several decades. This chapter discusses seven outcome studies in major brain injuries.
Short Historical Note 52
The Glasgow Coma SCALE and Glasgow Outcome Scale
The Title of the Paper
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4.
Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–4.
Fig. 7.1
Title page
The Paper and the Times
It is in the nature of things that prolonged coma from a major structural injury to the brain may lead to poor outcome. However, the assessment of comatose patients is complex because it requires a detailed neurologic evaluation that includes a reliable history, neurologic examination, and also interpretation of laboratory tests, neuroimaging, and sometimes electrophysiologic studies [23].
The key findings of the neurologic examination can be encapsulated into practical scales that allow physicians to communicate with each other and with other health care workers more effectively. Ideally, a coma scale would include the bare minimum of the unconscious state, and grading a patient over time must indicate changes in clinical condition. The scale or score may also indicate prognosis, but any coma study necessarily would include a heterogeneous group of patients with structural brain injury, and thus likely only those with very low scores may do poorly no matter what the cause. Prognosis of coma from nonstructural injury is far more difficult to predict.
Coma scales originated in neurosurgical intensive care units. Charting neurologic status and physiologic functions at the bedside was common practice. The need for a clinical bedside tool was universal. Ommaya, a neurosurgeon at the National Institute of Neurological Diseases and Blindness in Bethesda, Maryland, developed one of the earliest systems in 1966—a comprehensive scoring system called a “vital sign card”. It later became briefly known at the Ommaya Coma Scale [48].
Before the publication of the Glasgow Coma Scale (GCS), (Fig. 7.1) coma scales were not systematically used and—in day-to-day practice—descriptions of coma were likely much less detailed. Neurologists or neurosurgeons would communicate a full neurologic examination, but other specialists would probably commonly use ambiguous terms such as unresponsiveness or somnolence.
Prognosis could be determined early on but more accurately after the patient had been resuscitated and had entered a relative stable clinical state. Furthermore, classification of disability had always been a problematic assessment with easy pitfalls and limitations. Before Jennett and Bond devised a practical scale that later became known as the Glasgow Outcome Scale (GOS) (Fig. 7.2) several scales had been devised in Europe that pertained to outcome in head injury survivors. Outcomes that had been described previously included categories such as “permanent invalid,” “slight sequelae,” “partially reintegrated,” “mental restitution,” and were all poor interpretations of a patient’s functional well-being or handicap. When traumatic brain injury databases became well organized, there was a demand for tools that could evaluate outcome and also could predict futility.
The Details of the Paper
In 1974, Teasdale and Jennett published “Assessment of Coma and Impaired Consciousness: A Practical Scale,” which was a “spin-off” of the National Institutes of Health-sponsored international studies on coma and prognosis of severe brain injury [37]. The first version of this scale was known initially as the “Coma Index” but soon became known as the Glasgow Coma Scale. The GCS was constructed mainly to improve communication between physicians and nurses when describing different states of impaired consciousness.
To provide a tool for repeated measurements that could be charted hourly at the bedside and to “restrict routine observations to the minimum,” Teasdale and Jennett excluded certain tests from the scale (e.g., brainstem reflexes) that they believed would be difficult for “inexperienced junior doctors or nurses” to perform or interpret. The GCS, therefore, assessed only eye motor, and verbal responses. Teasdale and Jennett’s reasoning for the use of these three components was as follows:
Motor responses: “The case with which motor responses can be elicited in the limbs, together with the wide range of different patterns which can occur, makes motor activity a suitable guide to the functioning state of the central nervous system.”
Verbal responses: “Probably the commonest definition of the end of coma, or the recovery of consciousness, is the patient’s first understandable utterance.”
Eye responses: “Spontaneous eye opening, with sleep/wake rhythms, is most highly scored on this part of the scale, and it indicates that the arousal mechanisms in the brainstem are active.”
The GCS was initially an unnumbered system. The practice of assigning a number to the responses (using “1” for the lowest score rather than “0”) was introduced in a later article that also expanded the motor responses, adding abnormal flexion [70].
Meanwhile, the new computer database on traumatic head injury in Glasgow would need a standardized outcome assessment. Jennett and Bond’s paper on what became known as the Glasgow Outcome Scale was basically was a description of the nature of disability in survivors of brain damage. The GOS divided outcome into death or survival and in surviving patients whether there was a persistent vegetative state, severe disability, moderate disability, or good recovery. The GOS was used in their intensive care unit for some years, and they found “a good measure of agreement between different assessors.” No formal studies were presented in this paper, but its publication was a landmark development because it pointed out limitations of existing systems, summarized the difficulty in judging disability, and proposed a solution. The authors clearly emphasized that, when assessing disability, there is a patient component and a physician component. In other words, the patient may feel he is markedly handicapped while the physician feels that the patient is doing quite well considering the severity of his injury. The authors also acknowledged that the disability might be underestimated and a routine encounter with the patient may not reveal the extent of the cognitive deficit. Death requires no further description, but the authors were careful in determining that death could be ascribable to primary brain damage or, for example to severe pneumonia or sepsis. However, there was no clear distinction in the GOS between brain death, death from systemic complications or withdrawal of support in a patient with a major complication. The more difficult categories to assess were severe and moderate disability. Severe disability indicated that patients were dependent on daily care. Patients with moderate disability were independent and could travel alone, although a major handicap might be present. The good recovery category usually was seen as “resumption of normal life,” with minor neurologic or psychological deficits. The authors also had the fortitude to describe that return to work was a poor index of recovery. Moreover, the distinction of whether the patient will be institutionalized or home often had more to do with the support of the family rather than the disability.


Fig. 7.2
Title page
The Message and Acceptance
Although users of the GCS began creating sum scores for the three components (giving a total range of 3–15 points), this method was never the intention of the originators of the scale: “…while we do not favor its use in day-to-day clinical practice, we find no reason to doubt that it will continue to be used widely in the analysis and reporting of a series of patients with head injury or other forms of acute brain damage.” GCS sum scores such as 3, 8, and 15 have immediate familiarity and use of the sum scores even led to the slogan, “Glasgow 8, intubate.”
There have been criticisms over the years [28, 61]. First, GCS is skewed toward the motor part (six items vs. four for eyes and five for verbal). Second, the verbal component of the GCS is unusable in intubated patients. Several techniques and mathematical models have been devised to circumvent this problem. One technique is pseudoscoring, adding the average value of the motor and eye score to the sum in place of the missing verbal score. Another study using linear regression model found that the predicted verbal response correlated well with the actual verbal score, but the model is limited to patients with high GCS sum scores and nonintubated patients [59].
In clinical practice, most institutions substitute T (for “tube”) in the verbal component, but it is not clear how sum scores are calculated using this substitution. These attempts over the years to modify or simplify the GCS may be indicative of dissatisfaction with its use in patients with severe brain injury. Several other alternative coma scales have been proposed [65, 66, 68, 83]. None of the new coma scales have yet proven to be able to stand the test of time.
Additionally, when GCS is tested, physicians’ knowledge of the GCS is marginal. On average, when asked to name the individual scale components, physicians could identify three of the four possible eye responses, three of the six motor responses, and two of the five verbal responses. Approximately half of the neurologists and internists did not accurately name the verbal response [55].
The outcome scale became known as the GOS. (In a follow-up article, it was briefly called the Glasgow Global Outcome Scale [11].) The GOS was less easily accepted than the GCS, and many other scales have been developed. Nonetheless, the contribution was quite significant with dividing disability into being dependent (assistance with personal needs) and independent (able to look after oneself). It also included persistent vegetative state as a condition that should be separately recognized.
The GCS and GOS became adopted by most European countries and, through the ambassadorial work of neurosurgeon Langfitt, became a frequently used scale in the United States [58]. The scales would become universal metrics for severely brain injured patients. In his paper in 1978, Langfitt forcefully stated:
The author believes that the Glasgow Coma Scale and the Glasgow outcome measures should be adopted by neurosurgical units throughout the world to evaluate their patients with head injuries. Since other coma scales have merit, and changes such as the recent addition of a 15th point undoubtedly will be made in the Glasgow Coma Scale, there should be agreement to adopt the Coma Scale for a specified period of time, perhaps a 5-year period from 1978 to 1982. During that time, the applicability of the Coma Scale can be thoroughly tested and suggested changes catalogued in a central location [58].
The GOS has remained a preferred outcome system in assessing patients with traumatic head injury, particularly in Europe and the United Kingdom. Two decades later, Teasdale et al. published an extended GOS (8-point version) [71].
Nevertheless, the original GOS is still used in the two major traumatic brain injury data banks (The IMPACT and CRASH). The Cerebral Performance Category (CPC) and Rankin Scale also are used although they are basically modifications of the GOS. The CPC most often is used in studies of anoxic-ischemic encephalopathy. The Rankin Scale is most often used in studies on stroke. Albeit appropriately validated, the instruments that assess clinical outcomes vary considerably among studies and even vary in studies with the same patient populations.
Short Historical Note 53
Outcome After Nontraumatic Coma
The Title of the Paper
Levy DE, Bates D, Caronna JJ, et al. Prognosis in nontraumatic coma. Ann Intern Med. 1981;94:293–301.
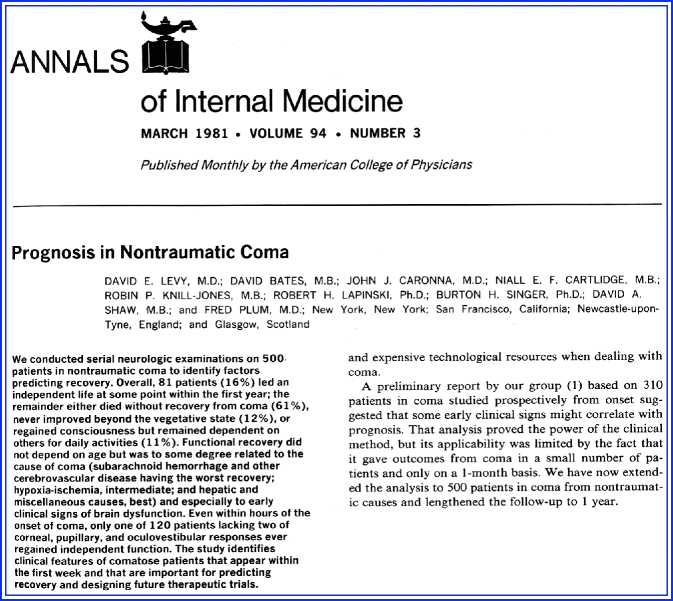
Fig. 7.3
Title page
The Paper and the Times
In the early 1960s, intensive care units (ICUs) were mushrooming in the United States and throughout the rest of the world. As a result, a discussion on medical costs and, in particular, high ICU costs, prompted a review of care in patients with hopeless neurologic conditions. Often, these discussions focused on the pressing issue of how to approach the long-term care of persistently comatose patients. Prognosis of acute neurologic conditions was nothing more than an educated empirical guess. Usually, a combination of persistent coma, abnormal pupil reflexes, and reflexive motor responses in a demonstrable severe brain injury would indicate a poor outcome, but guidelines had not been developed. Cohorts of comatose patients after traumatic brain injury had been published, but prognostic information about “nontraumatic” coma was not available. In these times, it was fashionable to longitudinally study a large number of patients with repeated neurologic examinations and then trying retrospectively to obtain useful predictors of outcome [19, 20]. The early studies mostly involved comatose patients following cardiopulmonary resuscitation (CPR) [47, 64, 88]. This study would involve all patients in nontraumatic coma. The study was a UK–US collaboration and a result of deliberations between neurosurgeon Bryan Jennett and neurologist Fred Plum [87].
The Details of the Paper
The study is an extension of the Bates paper that originally included 310 patients [7]. Levy’s paper has been better referenced due to its inclusion of useful algorithms. The study followed 500 patients in nontraumatic coma seen in New York Hospital—Cornell Medical Center, The Royal Victoria Infirmary (New Castle upon Tyne) and San Francisco General Hospital. Patients were identified by the investigators retrospectively while attending neurology rounds, visiting intensive care units and emergency departments, and daily for 3.5 years (Fig. 7.3). Patients in coma could be enrolled if coma lasted at least 6 h. Patients were followed daily to 1 month. The patient population was a mix of disorders such as subarachnoid hemorrhage (n = 38), cerebrovascular disease (n = 143), hypoxic-ischemic encephalopathy (n = 210), hepatic encephalopathy (n = 51), and a variety of other disorders (e.g., metabolic disturbances, infectious mass lesions). Outcome was determined by the Glasgow Outcome Scale.
The authors were able to determine outcome in certain subsets of patients. As expected, there was worse outcome in structural brain injury and best outcome in metabolic derangements causing abnormal level of consciousness.
The study found that the absence of pupillary light responses, corneal reflexes, and oculovestibular responses were each associated with a very low probability (<5%) of independent outcome (Fig. 7.4). But, no single sign predicted good outcome.
Clinical signs were then grouped in those seen at days 1, 3, and 7 and analyzed. On the first day, absent pupillary light responses or corneal reflexes predicted a poor outcome. Patients who would speak, opened eyes to noise, fixated on the examiner or were orienting—not unexpectedly—would do well.
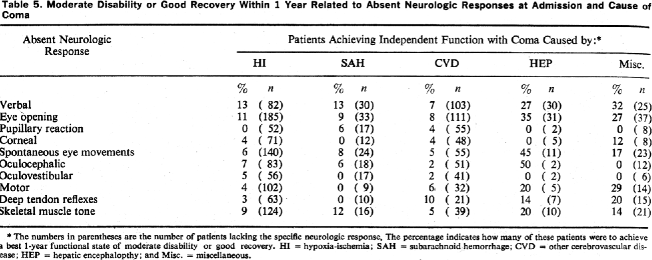

Fig. 7.4
Data on subcategories
At day 3, no patient recovered with absent pupil responses to light, no corneal reflexes, or no motor responses. At day 7, any absent brainstem reflex would indicate poor outcome. The data were then summarized in algorithms shown in Fig. 7.5.
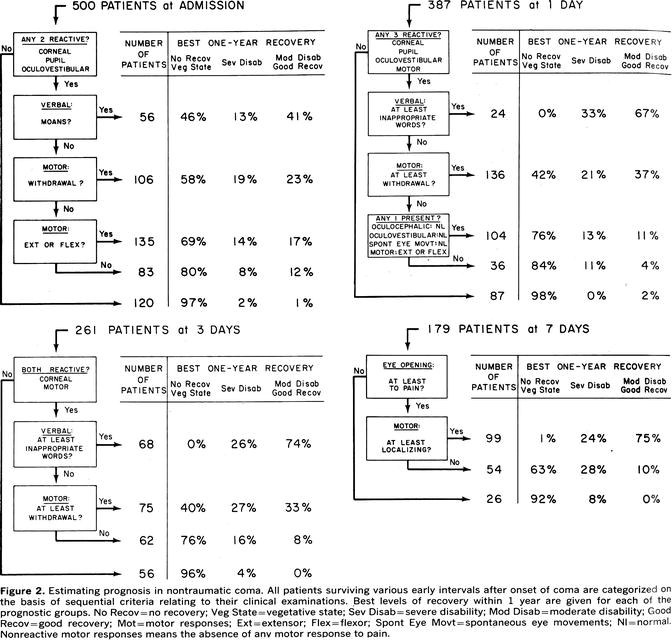

Fig. 7.5
The outcome algorithms
The paper also reported some other interesting findings. The data also showed a major difference between UK and US patients with 71% and 46% of patients, respectively, dying within the first 3 days. The study found that 24 patients with a (retrospectively determined) poor prognosis still survived more than 200 hospital days, indicating a reluctance of physicians and families to withdraw support.
The Message and Acceptance
Despite lack of computed tomography scan data, lack of details on level of care, lack of details in specific disease groups (i.e., cerebrovascular categories), and very wide confidence intervals of the reported percentages (due to small groups of patients in each prognosis category), the algorithms became a popular tool for neurologists. How they have influenced practice is not known, but pupil, corneal, and oculocephalic responses now were part of the clinical tools to assess the degree of coma and outcome probability. The study, however, eloquently found that assessment of injury to the brainstem was crucial in order to prognosticate accurately.
With better description of the structural injury and subclassification, the prognostic rules changed and became more refined. Therefore, an absent corneal reflex is more important in a massive hemispheric hemorrhage than in a cerebellar hematoma. The rules of prognostication may also be different in patients who are aggressively resuscitated or become neurosurgical candidates.
Very few studies have addressed outcome after nontraumatic coma, and most have concentrated on prognostication of coma after CPR. Recently, a study group performed an evidence-based comprehensive review on prognostication in comatose survivors after cardiac arrest and that resulted in new guidelines endorsed by the American Academy of Neurology. It became quickly clear by the authors writing this guideline that studies on prognostication had flaws and after careful selection criteria only 22 papers were selected. The definitions of coma and outcome were Glasgow Coma Scale score sum score 8 or less, “persistent unresponsiveness,” and “not regaining consciousness.” Poor outcome was defined as death or persisting unconsciousness after 1 month or death, persisting unconsciousness, or severe disability requiring full nursing care after 6 months.
Only a few reliable prognosticators of poor outcome after cardiopulmonary resuscitation were identified; if they were present, severe disability, persistent vegetative state, or death was very likely (defined as a false-positive rate approaching zero) [85].
The current practice has evolved over the years, but neurologists use the following basic principles of prognostication in comatose patients after CPR:
Exclusion of confounders such as prior use of sedatives or neuromuscular blocking agents, hypothermia, and presence of organ failure (including shock).
Identification of possible loss of all brain function and consideration of brain death.
Identification of key poor prognostic signs such as myoclonus status epilepticus, absent pupil or corneal reflex after 72 h from resumption of circulation, or a motor response not better than extensor responses.
Identification of abnormal laboratory tests such as increased neuron-specific enolase (NSE) or absent cortical potentials on somatosensory evoked potential studies.
These indicators of poor prognosis in comatose survivors are derived from studies in patients not treated with therapeutic hypothermia. Assessment of clinical markers—particularly motor responses—is more difficult due to possible prolonged effects of sedation after hypothermia [25]. Moreover, the prognostic value of a single sample of serum NSE is very uncertain. Serial measurements showing an increase may have more value but this remains to be proven [24]. Failure to awaken several days after CPR may prompt magnetic resonance imaging (MRI), but its value as an independent predictor is not yet established. Finding quantitative MRI evidence of diffuse laminar cortical necrosis in an unchanged comatose patient obviously predicts a poor outcome but MRI may remain nearly normal initially in patients bound for longterm nursing care.
Short Historical Note 54
Outcome after Traumatic Brain Injury
Title of the Paper
Jennett B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual patients after severe head injury. Lancet. 1976;1:1031–4.

Fig. 7.6
Title page
The Paper and the Times
The agony of a family suddenly confronted with severe traumatic brain injury (TBI) starts early. Family members often enter an ambivalent period of waiting and hoping. Some comatose patients are stable, but many require aggressive management of increased intracranial pressure (ICP) and that may include decompressive craniectomy. Even in the most aggressively treated patient, the outlook is uncertain, perhaps acceptable, hopefully not too grim, but rarely detailed enough to make radical decisions on level of care early on.
As early as the 1970s, articles from Scandinavian countries claimed to have identified clinical features that would portend poor outcome [15, 49]. Prediction of good outcome has always been tentative because certain major complications (i.e., pulmonary embolus, sepsis) could not be predicted. The Scandinavian studies focused on patients awakening from coma and the probability of them being able to return to work or care for themselves. No systematic studies on detailed neurologic findings linked to outcome categories in large population were known until Bryan Jennett’s effort to study severe TBI in Glasgow (Scotland), Rotterdam and Groningen (The Netherlands) (Fig. 7.6).
The Details of the Paper
A database of 600 prospectively collected patients was used to calculate predictions of outcome. Outcome was defined at 6 months and with clinical observations on days 1–7. The clinical examination included pupil responses, eye movements, and motor responses. The outcome categories included death, persistent vegetative state (PVS), severely disabled, moderately disabled, and good recovery as defined in the Glasgow Outcome Scale.
The study importantly stated that “no individual predictions have been made in a prospective fashion while the patient was still under treatment.” Additionally, the authors stated that “the patients in each of the centers were treated by a considerable number of neurosurgeons and neurologists who were not involved in the investigation and who were not asked to modify their practice.”
The authors considered it a “preliminary report.” The outcomes were distributed similarly during the 8 years of the study, making it less likely that patient care was influenced by the study. Bayesian probability statistics were used. A confident prediction was considered with a probability of 0.97 or more. The authors selected 200 existing patients at random, and outcome was predicted after comparison with the remainder 400 cases in the series. The study results found that confident predictions were made in 44% of patients seen within 24 h and this increased to 52–61% with data obtained after 3 days.
The study identified several factors associated with outcome. These were age over 20 years with increasing mortality beyond this cutoff age and even more likelihood of morbidity after 60 years. No differences were found between the decades. A major correlation was found between outcome and eye movement abnormalities (95% of patients with absent eye movements died or remained in PVS), pupil responses (95% of patients with absent pupil reflexes died or remained in PVS), and motor responses (83% of patients with extensor responses died or remained in PVS). The presence of an intracranial hematoma—whether left or right—was associated with a worse outcome.
The authors also identified “optimistic errors” as a result of “unexpected complications” in three patients. These were patients predicted to have a good recovery, but died or were left disabled.
The Message and Acceptance
The foresight of neurosurgeon Bryan Jennett to foster an international head injury databank and carry it through to a large data set remains a seminal moment in the understanding of TBI. The paper was the first to use mathematical calculations for predicting outcome. The authors were quick to point out that these predictions could only assist physicians and noted that hospital administrators should not use the data to guide the use of resources. The study’s main message, however, was that severe TBI often resulted in a poor outcome.
The study spawned a major increase in TBI databanks throughout the world [13, 41, 42, 44, 67]. Gradually, the number of clinical predictors increased, and computed tomographic (CT) data were added—largely unavailable in Jennett’s series [44, 46]. Systemic variables (hypotension, anemia) also became recognized as possible predictors.
Any predictive model recognizes pitfalls. Percentages can be “read” both positively and negatively, but these models are definitively better than clinical judgment based on experience. No physician can claim to have sufficient clinical acumen as to be absolutely certain. Poor outcome is more difficult to predict in younger patients [39, 40].
The most important factors with high predictive power are coma after adequate resuscitation, age of 65 years, abnormal pupils or abnormal pupils for at least one observation, shock on admission, persistently increased ICP, hypoxemia on admission, and CT scan abnormalities representing multiple contusions.
Currently, multiple prediction models in head injury exist. One of the largest databases of TBI (IMPACT; International Mission for Prognosis and Analysis of Clinical trials in Traumatic brain injury) uses admission characteristics to calculate a prognosis estimate. These are age, motor response, pupil responses, presence of hypoxia and hypotension, CT categorization into severity of lesions and presence of a mass lesion, presence of traumatic subarachnoid hemorrhage, epidural mass, and also serum glucose and hemoglobin values (calculator at www.tbi-impact.org) [44]. Another database is the CRASH prognostic model (calculator at www.crash.lshtm.ac.uk) [46].
CT categorization now plays an important part in calculation of risk for poor outcome [78]. Shear lesions frequently are found on CT scans in patients with diffuse closed head injury. They commonly are located in the frontal cortex and white matter interface, basal ganglia, thalamus, and internal capsule. Shearing abnormalities of the corpus callosum often are associated with small lesions in the thalamus, splenium, or septum pellucidum, consistent with a fronto-occipital direction of acceleration.
The Marshall CT classification introduces CT features that may be important cutoff points. In this classification, a high- or mixed-density mass lesion implies contusion or hemorrhage. The extent of the lesion is determined by its volume, the cutoff being 25 cc. The higher risk for raised ICP is determined by present, absent, or compressed basal cisterns and the degree of midline shift—the cutoff point being 5 mm. These pathologies fall into the classes based on the severity:
Grade 1: No visible intracranial pathology.
Grade 2: Cisterns present with midline shift 0–5 mm; no high- or mixed-density lesion >25 mL.
Grade 3: Cisterns compressed or absent with midline shift 0–5 mm.
Grade 4: Midline shift >5 mm or surgically evacuated lesion and nonevacuated high- or mixed-density lesion >25 mL.
Grade 5: Any lesion surgically removed.
Grade 6: High- or mixed-density lesion >25 mL not surgically removed.
The main aim of the Marshall classification was to identify those TBI patients who are at higher risk for deterioration or death [43].
Prognostic features on magnetic resonance imaging scans have not been systematically studied in large groups of patients, but one study claimed a very poor outcome when corpus callosum lesions appeared in conjunction with brainstem lesions [42].
Short Historical Note 55
Outcome after Cerebral Hemorrhage
The Title of the Paper
Tuhrim S, Dambrosia JM, Price TR, et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–63.
Fig. 7.7
Title page
The Paper and the Times
Stroke (called cerebrovascular accident in the 1950s) is mostly understood as ischemic injury caused by occlusion of a large cerebral artery or as a intracerebral hemorrhage (ICH). Early studies were hampered by lack of angiographic verification, lack of computed tomography (CT) scans, reliance on autopsy confirmation, as well as other imperfections such as a careful neurologic examination over time.
In the 1980s, studies on outcome started to appear, mostly to obtain a better sense of the natural history [22, 29, 50]. These studies could then be a benchmark toward the evaluation of medical or surgical treatments. For example, better insight in prognostic factors could address the potential benefits of surgical evacuation of a cerebral hematoma. Furthermore, outcome studies could identify causes of worsening that could lead to its better recognition. It was also recognized early on that the natural history of acute stroke also may be biased by referral patterns and in some hospital series including the more severely affected patients. This prompted the development of studies of patients from a defined geographic location (“population-based studies”).
Medical complications only recently received attention. Modern intensive care unit care undoubtedly impacted on outcome, and a better outcome became apparent when patients with cerebral hemorrhage were transferred to more secure settings, rather than being simply observed on the ward.
The complex interaction of many factors could make studying the natural history an almost futile undertaking. Most studies of patients with a recent cerebral hemorrhage identified that the neurologic examination of the patient and initial clot size (more than 50 mL) was a potent predictor of outcome. The initial studies undoubtedly used heterogeneous groups; later studies were far more location specific, in particular when CT scans became available in the early 1970s. Limited deficits in a patient with a presumed ischemic stroke could now be linked to a hemorrhagic stroke, and the epidemiology of stroke changed as a result of that. The study cited here was an important first attempt to sort out the melee of factors that could contribute to outcome in cerebral hemorrhage (Fig. 7.7).
The Details of the Paper
The data were obtained through the pilot phase of the stroke databank, a cooperative effort of four hospitals and the National Institute of Neurological Disorders and Stroke [76]. The 30-day survival status of 82 patients with cerebral hemorrhage was the basis of this study. Tuhrim et al. identified multiple variables that were possibly related to ICH outcome. He included medical history (21 variables), demographics (11 variables), stroke course (8 variables), physical examination (4 variables), neurologic examination (20 variables), and laboratory values (7 variables) [75]. All variables that were statistically significant were entered into a multiple logistic regression model.
Factors associated with outcome were Glasgow Coma Scale (GCS) score, systolic blood pressure, vertical gaze palsy, severity of weakness, presence of brainstem–cerebellar deficits, and clinical course and size of parenchymal hemorrhage. The major prognostic factors included GCS (≤8), size of hemorrhage (large, more than one lobe; small, less than half lobe), and pulse pressure (≤40, 41–65, or more than 65) (Fig. 7.8).
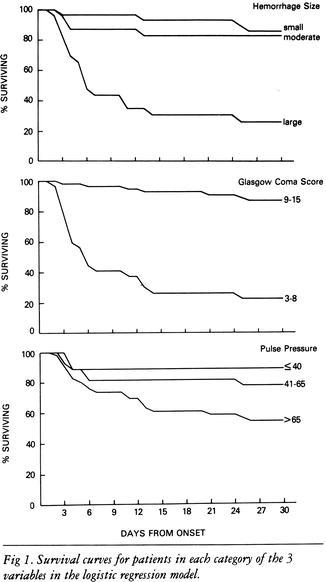

Fig. 7.8
Outcomes per variable
The Message and Acceptance
Application of Tuhrim’s model resulted in 90% correct prediction. The worst prognosis was seen in a patient with a GCS less than 8, hemorrhage involving more than one lobe, and a pulse pressure greater than 65 mmHg. The prognostic value of the pulse pressure was novel. (Pulse pressure is a surrogate of increased intracranial pressure). The model was later validated, now including hyperglycemia and intraventricular hemorrhage (IVH) extension as additional variables [50]. This time, IVH contributed significantly [77, 90].
Current ICH models have included GCS score, National Institutes of Health Stroke Scale, ICH volume, location (supra- or infratentorial), age, and presence of comorbidity. However, no universal scale has emerged and there remains a lack of a standard [16, 26, 30–32, 35, 57].
Further differentiation by specific lesion site was helpful in determining prognosis. Studies found that extension of the hemorrhage mattered greatly. For example, extension of a putaminal hemorrhage into the posterior limb of the capsule would markedly reduce chance of motor recovery [45]. Further classification would include lesions involving anterior limb of capsule and thalamus. Moreover, the significance of IVH depended on the location, with poor outcome in putaminal hemorrhage, but much higher chance of recovery in caudate hemorrhage. Presence of acute hydrocephalus (often a result of shift and compression by a large hematoma) also became recognized as a poor prognosticator in ganglionic hemorrhage with little improvement after placement of a ventriculostomy. Keeping the ventriculostomy open with thrombolytics-allowing clearance of intraventricular hemorrhage-may change the odds of a poor outcome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








