Fig. 19.1
(a) Colon NEC with a Ki-67 index of 30 %. (b) Colon NEC with a Ki-67 index of 90 %
NEC encompasses mainly two histological entities: small-cell neuroendocrine carcinoma (SCNEC) resembling small-cell carcinoma of the lung and a large-cell pleomorphic carcinoma (LCNEC) [5] (Fig. 19.2). LCNEC is morphologically distinguished from SCNEC by cytological features of a non-small-cell carcinoma, including large cell size, low nuclear-to-cytoplasm volume ratio, coarse chromatin and frequent nucleoli. There is small-cell histological preponderance in the squamous GI tract (oesophagus and anus) and large-cell carcinomas in the glandular parts [23]. Awareness of the latter is essential, as these tumours often are indistinguishable from poorly differentiated non-NET carcinomas. In a study on 87 high-grade GI tract tumours, no significant difference in survival was detected amongst small cell, mixed and large cell subtypes, nor were there any differences between tumours of the upper GI tact versus the lower GI tract [23]. In pancreatic NEC, small-cell NEC was genetically similar to large-cell NEC, and these genetic changes were distinct from pancreatic neuroendocrine tumours pNET [25]. There seems to be no significant differences in survival or response to chemotherapy based on histological subtypes [10, 21, 23]. The terms poorly differentiated high-grade NET and NEC have been used synonymously. However, recently data suggest that amongst large-cell NEC, there are tumours that are well differentiated [26, 27]. Survival was longer in well-differentiated NEC than in poorly differentiated NEC (40 months vs. 17 months) [26]. The presence of well-differentiated tumours in NEC needs to be validated in larger series, as it may have consequences for future classification and treatment.
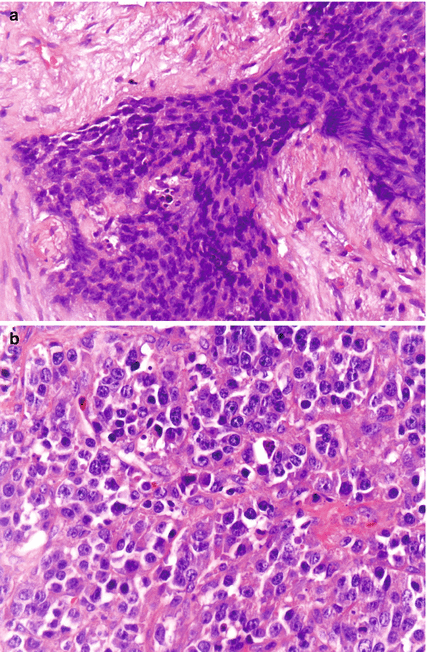

Fig. 19.2
(a) Small-cell NEC from pancreas. (b) Large-cell NEC from colon
NEC is characterised by immunohistochemical markers of neuroendocrine differentiation with synaptophysin, neuron-specific enolase, chromogranin A (CgA) and CD56 being the primary stains. Several pathologists advocate for routine staining with synaptophysin and CgA for all tumours initially classified as poorly differentiated gastrointestinal adenocarcinoma. Usually synaptophysin immunohistochemistry staining is positive, whereas staining for CgA may be negative. A strong positive staining for CgA was found in 45 % of patients with advanced GI-NEC, but the intensity of CgA staining was not correlated to survival or location of the primary tumour [10]. It is debated if a positive staining for synaptophysin or CgA is necessary to classify the tumour as an NEC. The presence of CgA indicates a more mature tumour, and the presence of both markers may be a good prognostic sign [11, 28]. In a study of 39 colorectal NEC patients, CD177 immunoreactivity (a stem cell marker) was found in 54 % of the tumours and was together with vascular invasion an independent prognostic marker for survival [24]. Achaete-scute homolog 1 has been proposed as a diagnostic marker of poor differentiation and may help to differentiate NEC from NETs in difficult cases [29].
19.3.2 Diagnostic Procedures
Diagnostic workup should include a CT scan of the abdomen and thorax. Location of metastases at the time of diagnosis of advanced disease in 305 NEC patients was in the liver (68 %), lymph nodes (62 %), lung (15 %), bone (15 %), brain (4 %) and other locations (27 %) [10]. PET is usually positive in NEC and could be of prognostic value [30]. PET/CT may be especially indicated for patients with apparently localised disease, to locate possible metastatic disease and avoid unnecessary surgery. CgA measurement has been assumed to be normal in GI-NEC patients; however, CgA was recently shown to be elevated in two third of 188 patients with advanced GI-NEC disease [10]. CgA could therefore be a useful tumour marker for NEC patients. Neuron-specific enolase has only been tested in patients with mainly pulmonary NEC [31].
The relevance of somatostatin receptor scintigraphy (SRS) in NEC patients is uncertain. In 14 patients with a Ki-67 >15 %, 69 % had a positive SRS [32]. In another study a high uptake on SRS was found in 69 of 182 patients (37 %), but SRS was less frequently performed in patients with a high Ki-67 [10]. A high uptake on SRS was more often found in primary pancreatic tumours (46 %) compared to primary tumours from other locations (0–25 %). In two small second line studies, 20–40 % of NEC patients had a high uptake on SRS [11, 12]. An SRS may be clinically relevant if the Ki-67 is below 40 %, as peptide receptor radionucleotide therapy (PRRT) then may be a treatment option [33–35]. Routine measurement of U-5HIAA does not seem meaningful, as hormone-related symptoms are rare and urinary 5-HIAA elevated in few patients [10].
Several baseline laboratory abnormalities are correlated with poor survival in NEC: low haemoglobin level and elevated levels of platelets, white blood cell count, lactate dehydrogenase (LDH), alkaline phosphatase and C-reactive protein (CRP), with LDH and platelet count as the strongest factors [10]. It is increasingly recognised that variations in outcome are not solely determined by the characteristics of the tumour, but also by host response factors. Markers of a systemic host inflammatory response, e.g. elevated platelets and leucocytes, have a prognostic value in a variety of common solid tumours [36]. LDH may be a biomarker for hypoxia and highly angiogenetic tumours, and high LDH-5 is directly related to an up-regulated Hypoxia-induced Factor (HIF) pathway and linked with an aggressive tumour phenotype [37].
19.4 Treatment
Treatment of this very aggressive disease remains a challenge for clinicians. NEC is characterised by a high proclivity for metastatic dissemination even in patients with clinically localised tumours. Extensive disease is almost invariably treated by systemic chemotherapy. In contrast, the treatment approach for limited disease, a potentially curable condition, is presently neither consistent nor uniform. Retrospective studies have shown that surgery alone is rarely curative [7]. Based on the treatment paradigm for limited-stage small-cell lung cancer, chemotherapy and local therapy (surgery or radiation) can be considered in many patients with locoregional EP-NEC. Adjuvant or neoadjuvant chemotherapy is usually given with cisplatin/carboplatin and etoposide, based on the effect seen in metastatic setting.
19.4.1 Surgery and Neoadjuvant (Perioperative) Treatment
Despite rare reports of long-term survivors, surgery alone is usually inadequate therapy even for apparently localised disease. Adjuvant radiotherapy and chemotherapy are widely recommended, although the effectiveness of a combined modality approach has not been firmly established. Casas and colleagues highlighted the role of systemic chemotherapy and local treatment in oesophageal SCC. In a retrospective series of 199 patients, improved survival from 5 to 20 months was reported in patients with localised disease who received multimodal therapy, i.e. surgery, radiation therapy and systemic treatment [38]. Local therapy only (e.g. surgery alone) was the single most powerful predictor of poor prognosis, whereas chemotherapy was strongly associated with improved survival. Median survival was 20 months after induction chemotherapy followed by consolidative chemoradiation for 25 patients with limited disease oesophageal SCNEC [39]. Surgery might therefore not be necessary as part of initial therapy for oesophageal NEC if complete response is achieved after chemoradiation. A study on GI SCC however suggests a potential role for surgery for limited disease with pre- or postoperative chemotherapy, as half of the operated patients retained locoregional control [15]. Altogether, most data support that with limited disease, a combination of systemic platinum-based chemotherapy with local treatment consisting of radiotherapy, surgery or both offers the best chance for long-term survival. Several authors suggest that a sequence of neoadjuvant chemotherapy followed by definitive surgery seems appropriate [8]. Potential advantages to a neoadjuvant approach include earlier treatment of micrometastatic disease, better compliance to treatment, determination of responsiveness to chemotherapy and avoidance of unnecessary surgery for patients with early systemic disease progression.
In contrast to metastatic NETs with a low Ki-67, debulking surgery and surgery for liver metastasis are generally not recommended in NEC patients. Surgery or locoregional treatment for metastatic disease after neoadjuvant chemotherapy can however in selected patients give long-time survival (Fig. 19.3) [40, 41].
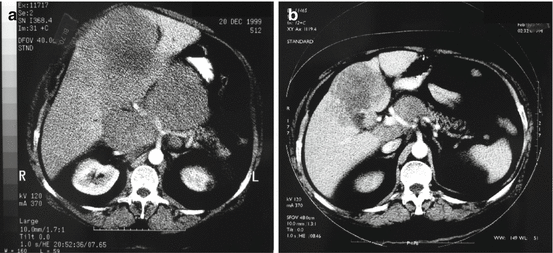

Fig. 19.3
Baseline (a) and partial response of liver metastases and lymph node metastases after 4 months of neoadjuvant chemotherapy with cisplatin/etoposide (b) before radical surgery and long-term survival
19.4.2 Postoperative Adjuvant Chemotherapy
Although there are no studies examining postoperative adjuvant chemotherapy in NEC, their aggressive behaviour warrants consideration of adjuvant therapy in most cases. The North American Neuroendocrine Tumor Society (NANETS) recommends 4–6 cycles of cisplatin or carboplatin and etoposide as adjuvant therapy for resected patients [1]. Several treatment schedules are recommended by the National Comprehensive Cancer Network (NCCN) and the European Neuroendocrine Tumor Society (ENETS) [42, 43]. A commonly used schedule is etoposide 100 mg/m2 for 3 days and cisplatin at a dose of 45 mg/m2/day on days 2 and 3, every 4 weeks [43]. As rapid recurrence often occurs after surgery, postoperative radiological staging should be performed before initiation of adjuvant chemotherapy.
19.4.3 Palliative Chemotherapy
Advanced GI-NEC is an aggressive disease where rapid referral to an oncologist is necessary, some advocate referral within a week. Patients should be referred for possible treatment before the performance status (PS) deteriorates to the extent that that the patient is no longer fit to receive chemotherapy. Median survival in 53 patients not given chemotherapy was only 1 month after diagnosis of advanced disease [10]. Patients not receiving palliative chemotherapy were older and had a worse PS, less positive SRS, less intense CgA staining and more often elevated white blood cell count and C-reactive protein levels. The very short median survival for patients not receiving chemotherapy indicates that the benefit from palliative chemotherapy is probably substantial.
19.4.3.1 First-Line Chemotherapy
Guidelines for advanced extrapulmonary NEC advocate the use of platinum-based chemotherapy combined with etoposide [1, 42–44], although until recently only few and small first line studies have been reported. In the study of Moertel et al., 18 predominantly GI-NEC patients were treated with cisplatin/etoposide [45]. Etoposide was given as 130 mg/m2 iv days 1–3 and cisplatin 45 mg/m2 days 2–3 every 4th week. Median number of courses was 5, 67 % had objective tumour regression, time to progression was 8 months and median survival was 19 months. Mitry et al. treated 41 poorly differentiated NEC; 27 patients were GI-NEC or CUP with predominantly GI metastases [16]. They were treated with etoposide 100 mg/m2 days 1–3 and cisplatin 100 mg/m2 day 1 every 3rd week. Median number of courses was 4, response rate 42 %, complete response (CR) 10 %, partial response (PR) 32 %, and stable disease (SD) 34 %. Progression-free survival (PFS) was 9 months and median survival 15 months. Toxicity was however high probably due to the 1-day cisplatin schedule. A 3-drug regimen combining carboplatin/etoposide with paclitaxel was given to 78 patients with NEC with mainly unknown primary tumour [46]. Although response rate was high (53 %), median survival was only 14.5 months and as grade 3–4 toxicity was frequent this schedule is not widely used. Although treatment with cisplatin/etoposide often induces initial tumour reduction, progressive disease usually occurs rapidly (Fig. 19.4).
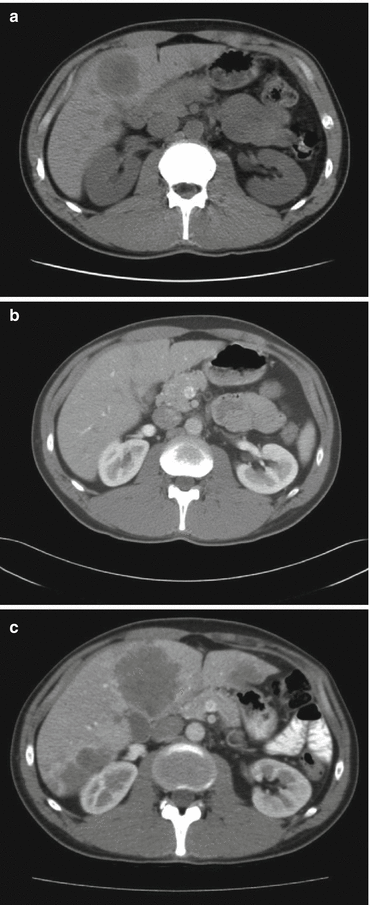

Fig. 19.4
Baseline (a) and partial response after 6 months of cisplatin/etoposide treatment (b), but massive progression of disease after a 2-month treatment break (c) in a 39-year-old patient with primary rectal NEC
Recent data suggests that response rates and survival of GI-NEC patients might be lower than previously reported. In a retrospective study palliative chemotherapy was given to 252 patients with advanced GI-NEC. They were mainly treated with cisplatin/etoposide or carboplatin/etoposide and some with carboplatin/etoposide/vincristine. The median number of courses was 4; response rate was 31 % (2 % CR, 29 % PR) and disease control rate 64 %. PFS was 4 months and median survival 11 months with 2- and 3-year survival of 14 and 9.5 %, respectively. No differences were seen according to chemotherapy schedule regarding response rates, PFS and survival (Fig. 19.5a), indicating that in GI-NEC cisplatin could probably be replaced by carboplatin. No further benefit was seen by adding vincristine to the etoposide/platinum combination. Multivariate analyses of the clinical and pathological features of this patient cohort identified several variables associated with response to treatment and survival such as performance status (PS), proliferation index, platelet count, lactic dehydrogenase levels and primary site of origin [10]. Median survival was only 8 months in primary colon tumours treated with chemotherapy, significantly shorter than the 15 months for patients with primary pancreatic tumours (Fig. 19.5b). Performance status was a strong prognostic factor for survival and varied from a median survival of 18 months with PS 0–5 months with PS 2 (Fig. 19.5c). Patients with PS 2 had a higher percentage of immediate disease progression compared to PS 0 (61 % vs.26 %). A relevant question is whether GI-NEC patients with a poor PS will benefit from a potentially toxic platinum-based treatment. However, in PS 2 patients who achieved a partial response after chemotherapy (25 %), PFS and median survival were 5 and 11 months, respectively. Patients with a PS 2 at the time of diagnosis should therefore not be excluded from receiving palliative chemotherapy. An ROC analysis was done in this study to investigate a possible correlation between response rate and Ki-67 level. The best cut-off value concerning response rate for Ki-67 was 55 %. Tumours with Ki-67 <55 % were less responsive to platinum-based chemotherapy (response rate 15 % vs. 42 %) (Table 19.1, Fig. 19.6). Patients with Ki-67 <55 % had a significantly longer survival compared to patients with higher Ki-67 levels (14 vs.10 months) and may thus constitute a different disease entity (Table 19.1, Fig. 19.7). Thirty months after chemotherapy initiation, 23 % of patients with a Ki-67 <55 % were alive compared to only 7 % when Ki-67 ≥55 %.
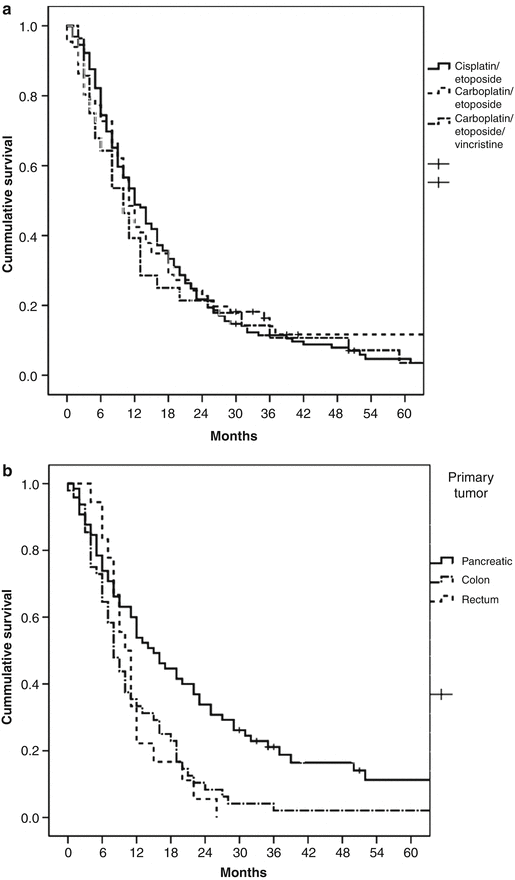
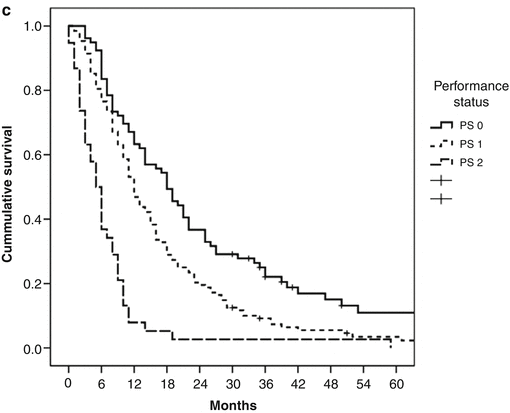


Fig. 19.5




Survival in patients treated with chemotherapy according to (a) chemotherapy schedule, (b) location of primary tumour and (c) performance status (From Sorbye et al. [10])
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






