10
Hemangiomas and Dural Fistulas
♦ Cavernous Malformations
Cavernomas or cavernous malformations of the central nervous system are uncommon pathologic entities, accounting for approximately 16% of all intracranial vascular lesions.1 Histologically, they are composed of a dilated, single endothelial cell layer and sinusoidal veins with little or no intervening neural tissue (Fig. 10.1).2 Ultrastructural studies have demonstrated that the absence of tight junctions between endothelial cells may account for the propensity of cavernous malformations to leech blood products into adjacent tissue.3 These lesions are usually angiographically occult because of their slow flow, which belies their proclivity for hemorrhage. Their magnetic resonance imaging (MRI) appearance is uniquely characteristic. T2-weighted images exhibit a dark, hemosiderin-stained rim and mixed-age blood products composing the nidus of the lesion. Patchy enhancement is observed occasionally, but otherwise cavernous malformations seldom enhance. Contrast administration usually unveils an associated venous angioma.
Posterior fossa cavernous malformations account for approximately 9 to 35% of intracranial cavernous malformations.4–6 In order of decreasing frequency, the most common locations involving these lesions are the cerebellum, pons, midbrain, and medulla.7 On rare occasion, cavernous malformations of the cranial nerves are discovered (Fig. 10.2).8 Symptomatic annualized event rates for brainstem cavernous malformations have been reported to range from 0.25 to 22.9%.9–11 It is believed that hemorrhage rates rise significantly after the initial hemorrhage, with rebleed rates reported as high as 30 to 60% per patient per year.7,12 Posterior fossa cavernous malformations, particularly in the brainstem, are associated with a significantly worse clinical course than lesions in supratentorial locations.5 In the analysis of our institutional series, which is based on the assumption that the lesions were present since birth, the retrospective hemorrhage rate was 5%.7 In another prospective study, the annual event rate for deep lesions was 10.6%.9 Hemorrhage rates have been reported higher in women, implicating estrogen as a risk factor.12–15 With the widespread availability of MRI, posterior fossa cavernous malformations are being detected more frequently and often incidentally.
Because they are usually located in exquisitely eloquent parenchyma, posterior fossa cavernous malformations present a particularly challenging tactical quandary for neurosurgeons. The stochastic nature of brainstem cavernoma hemorrhages is problematic. A phenomenon known as “temporal clustering” of brainstem cavernomas refers to the clustering of hemorrhagic events in a relatively short time period, flanked by longer periods of relative quiescence. These active periods involving multiple hemorrhages can produce a significant stepwise decline in neurologic function.16,17 Hence, an untreated patient who suffers repeated hemorrhages should be strongly considered for surgery. However, surgical intervention risks iatrogenic neurologic deficits and poses a daunting technical challenge in this unforgiving region of the brain. Hence, the treatment algorithm for posterior fossa cavernous malformations can be unclear.
In our experience, we base the decision to operate on the presence of the following factors: (1) neurologic symptoms directly attributable to repeated hemorrhages; (2) documented intra-or extralesional hemorrhage associated with mass effect; and (3) posterior fossa cavernous malformations that approach a pial surface, are exophytic, or are adjacent to non-eloquent parenchyma that serves as a surgical avenue. Surgical intervention on acute or subacute hemorrhagic lesions can take advantage of the plane created by the hematoma between the cavernous malformation and parenchyma, thus favoring early operation. However, patients are often referred after significant or even complete recovery from initial deficits. Therefore, the timing of surgery has varied, depending on the severity of a patient’s clinical presentation and on referral patterns.
Because the natural history of posterior fossa cavernous malformations is not completely understood and symptoms can change dramatically over time, a careful discussion of treatment options with patients and family members is mandatory. The option of expectant observation must be explicitly addressed.18 Asymptomatic lesions or previously symptomatic patients who have recovered from initial deficits may be followed nonsurgically, particularly if the cavernous malformation is deep-seated or minute in size. If surgery is recommended, patients and family should be informed that direct intervention typically mimics the course of a hemorrhagic event. That is, patients should expect to suffer transient, mild but discernible, neurologic deficits following surgery. Most patients, however, return to their baseline preoperative function during follow-up.7
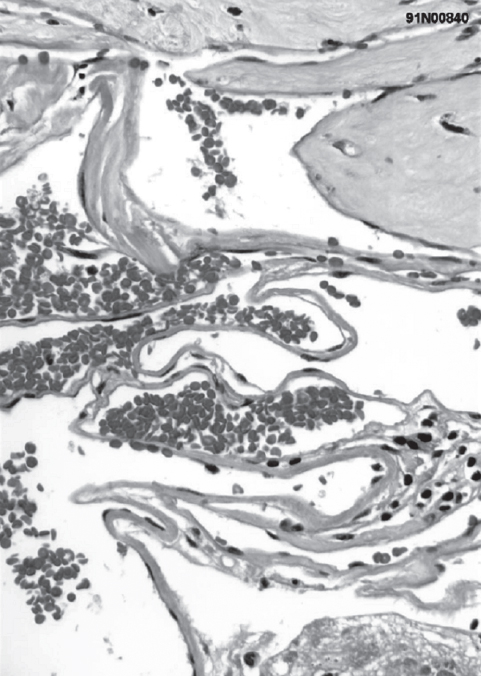
Fig. 10.1 Histology demonstrates dilated, sinusoidal veins and the absence of smooth muscle layers. (Courtesy of Barrow Neurological Institute.)
♦ Surgical Approach
Once the decision to pursue surgery has been made, the surgical approach is chosen by using the two-point method (Fig. 10.3).7,19 Extending a line from the center of the lesion through the nearest point of contact with a pial surface or surgical corridor demonstrates the optimal surgical approach. This method maximizes exposure and minimizes transit through eloquent brain tissue. For posterolateral midbrain lesions, we prefer either a subtemporal or supracerebellar-infratentorial approach.20,21 Anterior pontine lesions are accessible through a transsylvian, orbitozygomatic, or retrosigmoid corridor. Lower brainstem lesions are approached via a midline suboccipital or far-lateral craniotomy depending on their precise location (Fig. 10.4).
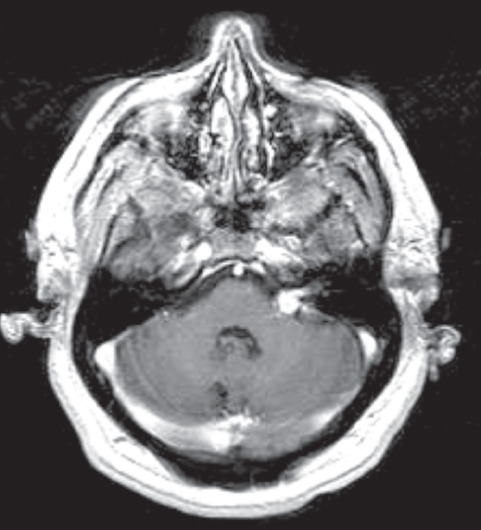
Fig. 10.2 Cavernous malformation involving the seventh and eighth cranial nerve complex was confirmed histologically. Numerous other cavernous malformations were present throughout the brain. Cavernous malformations involving the cranial nerves can have the typical heterogenous appearance on magnetic resonance imaging (MRI). Alternatively, as in this case, they can enhance and mimic a neoplastic process. (Courtesy of Barrow Neurological Institute.)
♦ Microsurgical Technique
After the appropriate approach has been selected, several important technical issues involving microdissection of posterior fossa cavernous malformations must be considered. For lesions that reach a pial surface, the malformation is easily visualized before surgical manipulation. A simple corticectomy can be performed directly over the lesion, followed by microdissection around its borders. We favor microdissectors (Synergetics USA, St. Louis, MO) that offer a variety of instrument angles and microcurette tip sizes to facilitate resection. It is imperative to maintain the plane around the lesion itself without violating the thin-walled, sinusoidal veins of the posterior fossa cavernous malformation or disrupting normal adjacent (usually hemosiderin-stained) brain tissue.
For lesions just beneath the pial surface or buried by overlying parenchyma, we routinely use neuronavigation (Stealth Station; Medtronic SNT, Louisville, CO) as an essential adjunctive tool for locating the lesion. Preoperative diffusion tensor imaging (DTI), which visualizes major white matter tracts, can aid in the selection of a surgical approach by determining which tracts are at risk during dissection and lesion removal.22,23 This technology can also be applied to intraoperative neuronavigation, with the hope of reducing postoperative neurologic deficits.24 Intraoperative nerve stimulation of the cranial nerve VII can be useful when treating lesions adjacent to the floor of the fourth ventricle.
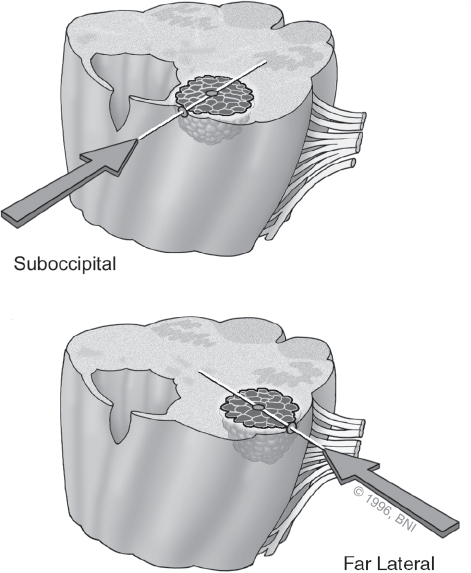
Fig. 10.3 Two-point method. A line connects the center of the lesions with the nearest point on the surface. This trajectory determines the optimal approach for resection. (Courtesy of Barrow Neurological Institute.)
For posterior fossa cavernous malformations deep to the pial surface, a fundamental understanding of access zones in the brainstem is required to enter lesions adjacent to eloquent brainstem structures while minimizing residual deficits (Fig. 10.5).25 We advocate an extralesional dissection and excision of the lesion when possible. If a cavernoma is entered inadvertently, the thin walls of the lesion will collapse, which renders them difficult to identify and dissect from the walls, and risks leaving residual malformation. Once the lesion has been resected, a thorough final inspection of the walls ensures that no residual remains. Bipolar cauterization for hemostasis is used only sparingly to minimize tissue damage. Procoagulant agents such as Nu-Knit (Johnson & Johnson, Arlington, TX) or FloSeal (Baxter Healthcare, Fremont, CA) are preferred.
Frequently, a venous angioma is encountered during microdissection (Fig. 10.6). This association is well described,26 and a popular theory places cavernous malformations, venous angiomas, and capillary telangiectasias along the same pathophysiologic spectrum.27 It is imperative to distinguish this entity from the cavernous malformations itself. Inadvertent injury or resection can lead to devastating venous infarction.
Typically, we obtain postoperative MRIs to serve as a baseline for future comparisons. A 1-year postoperative MRI is followed by regular scans at increasing intervals to monitor for the rare possibility of recurrence. After 10 years of follow-up, patients are advised to obtain imaging studies only if symptoms recur.
Our previously published surgical experience7 with 100 patients with brainstem cavernous malformations yielded 87% favorable outcomes, 9% worsening of neurologic deficits, and 4% mortality. These results are consistent with data from other series.4,28–30 Comparatively, our untreated cohort of 83 brainstem cavernous malformations have fared worse, with 65% favorable, 33% worse than at presentation, and 2% mortality.21 Permanent complications have ranged from 12 to 70%.6,7,29,31 These data stress the importance of careful patient selection before exercising surgical options. The surgical risks also must be weighed against the poor natural clinical history of brainstem cavernous malformations when compared with supratentorial lesions. Using judicious inclusion criteria and careful microsurgical technique, these lesions can be resected with acceptable results (Fig. 10.7).
♦ Radiosurgery
The effect of stereotactic radiosurgery on cavernous malformations remains controversial. Several studies have demonstrated a potential beneficial effect of radiation therapy, stereotactic proton-beam therapy, or stereotactic radiosurgery on the hemorrhage rate associated with cavernous malformations.29,32,33 Hasegawa et al34 documented a dramatic reduction in hemorrhage rate from 33.9% per year before gamma knife radiosurgery (GKRS; mean pre-GKRS follow-up of 4.33 years) to 12.3% per year in the first 2 years after GKRS. This rate dropped to 0.76% per year during the 2nd through 12th years of follow-up. In this series, the radiosurgical morbidity rate was 13.4%. Similarly, Pollock et al35 reported a significant reduction in the annual hemorrhage rate from 40% to 2.9% after 2 years of follow-up. However, their rate of treatment morbidity was 41%. Finally, a large series by Lunsford et al36 showed that patients with hemorrhagic or symptomatic lesions in deep or highly eloquent structures treated with GKRS had a significant reduction in hemorrhage rate from 32.5% to 10.8% per year for the first 2 years after treatment. The rate was reduced to 1.06% annually thereafter with up to 20 years of follow-up. Treatment morbidity was 13.5%.
Whether GKRS really affects cavernous malformations is confounded by multiple variables, including the effect of temporal clustering of hemorrhages on outcome after GKRS, relatively short follow-up periods, population or referral selection biases toward GKRS, and the lack of histologic evidence of radiation-induced changes. It is important to recognize that radiosurgery, particularly in the posterior fossa, subjects patients to a modest but significant risk of morbidity, whereas the effect on the lesion itself is only poorly understood. Future long-term follow-up studies may better elucidate the potential benefits of radiosurgery. Based on current evidence and our clinical experience, we do not recommend radio-surgery as a primary treatment option to our patients.
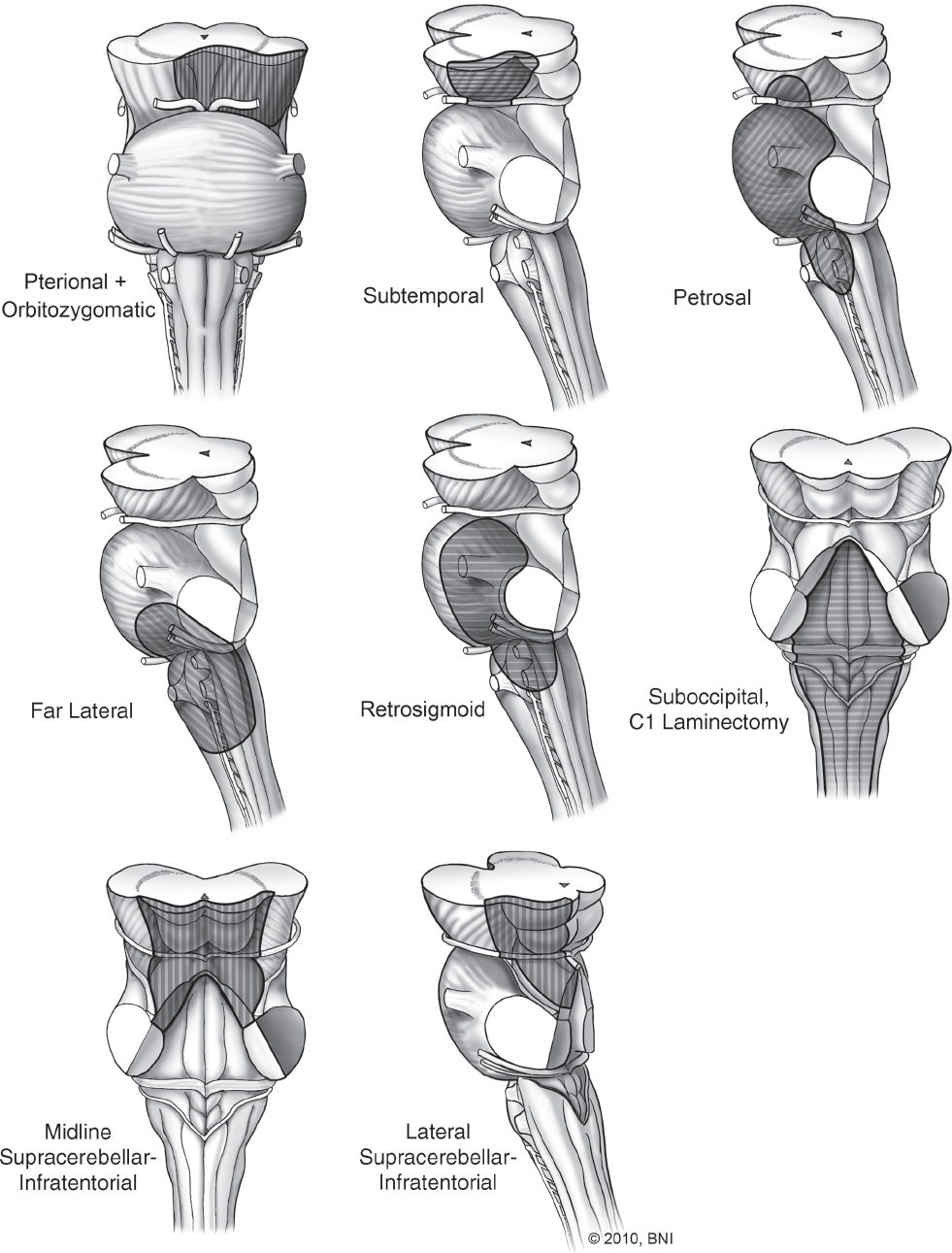
Fig. 10.4 Surgical approaches to the . brainstem. (Courtesy of Barrow Neurological Institute.)
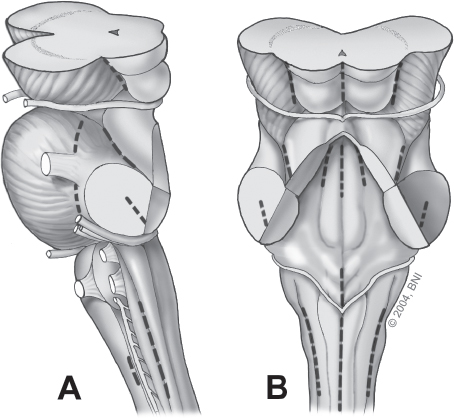
Fig. 10.5 Annotated lines indicate relative safe entry zones into the brainstem when cavernomas lie beneath the pial surface. (A) Lateral view. (B) Posterior view. (Courtesy of Barrow Neurological Institute.)
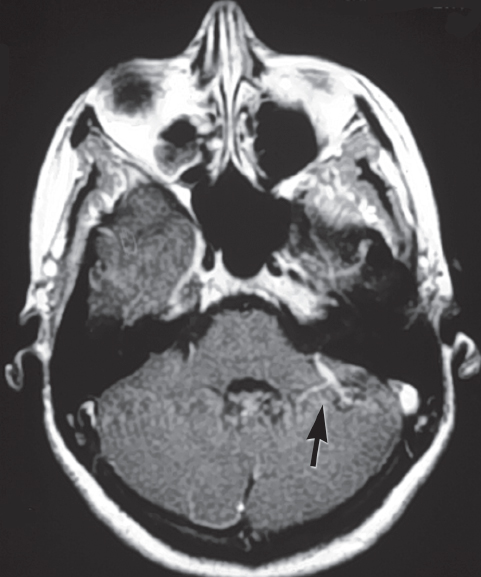
Fig. 10.6 MRI of a classic venous angioma associated with a cavernous malformation (arrow). (Courtesy of Barrow Neurological Institute.)
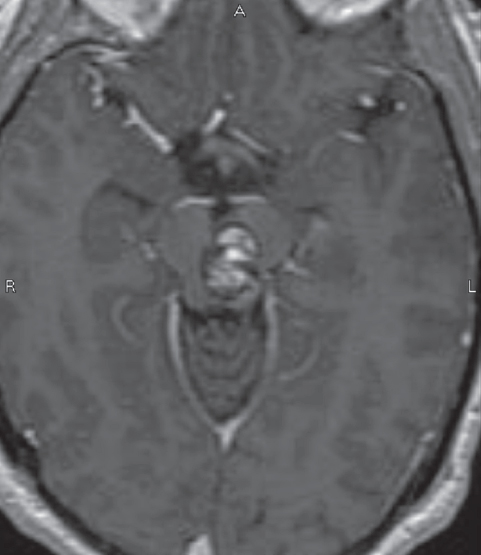
Fig. 10.7 Mesencephalic brainstem cavernous malformation. Two-point method favors either a subtemporal or lateral supracerebellar infratentorial approach; the latter was used in this case. A safe entry zone in the posterolateral midbrain, lateral to the tectal plate, was chosen as the entry point. (Courtesy of Barrow Neurological Institute.)
♦ Dural Fistulas of the Posterior Fossa
Like cavernous malformations, dural arteriovenous fistulas (dAVFs) are uncommon entities of the posterior fossa that can manifest incidentally, secondary to neurologic symptoms, or due to frank hemorrhage. They account for 10 to 15% of all intracranial vascular lesions.37–41 The transverse-sigmoid sinus (38%) is the most common location of dAVFs, followed by the cavernous sinus (34%). Less common locations include the superior sagittal sinus (5%), ethmoidal sinus (4%), superior and inferior petrosal sinuses (5% and 3%, respectively), and marginal sinus (4%).42,43
The most common symptom at presentation is headache. However, bruits, visual symptoms, venous infarction, and intracranial hemorrhage are well described.44,45 dAVFs are also associated with trauma, chronic venous hypertension, middle ear infections, surgery of the venous sinus, or sinus occlusion.42 Dural sinus thrombosis is strongly correlated with the pathophysiology of dAVF formation and persistence. Once the venous system is subjected to arterialized pressures from the fistula, ensuing venous hypertension can lead to leptomeningeal venous drainage, venous varices, and subsequent risk for hemorrhagic or ischemic events. The link with angiogenic growth factors has been described in animal models,32,46 the inhibition of which might serve as a potential treatment avenue in the future. Nonetheless, most patients have no antecedent history to explain the formation of the lesion.
A multivariate analysis of more than 100 cases47,48 demonstrated that leptomeningeal or galenic (deep) venous drainage pattern and venous varix formation significantly correlated with aggressive behavior and poor outcome. Using angioarchitectural features, grading scales have been formulated to predict the risk of neurologic symptoms or hemorrhage.49–52 The University of California–San Francisco (UCSF) scale describes four grades of dAVFs.49 Grade 1 lesions exhibit anterograde drainage via normal sinus pathways. Grade 2 lesions demonstrate antero-and retrograde venous drainage with or without cortical venous drainage. Grade 3 lesions have retrograde venous drainage related to an occluded sinus. Grade 4 lesions have only cortical venous drainage. According to the UCSF data, hemorrhage occurred in 31% of grade 3 and in 100% of grade 4 lesions.
The Cognard classification describes five types of dAVFs delineating location, direction of flow, and drainage patterns.50 Type 1 lesions have antegrade drainage into a dural venous sinus. Type 2 lesions are divided into three subtypes: type 2A lesions have direct retrograde drainage into a dural sinus; type 2B lesions flow antegrade into a dural sinus with retrograde cortical venous drainage; and type 2A+B lesions have retrograde flow into a dural sinus with retrograde cortical drainage. Type 3 lesions drain directly into nonectatic cortical veins. Type 4 lesions have direct ectatic cortical venous drainage. Type 5 lesions have retrograde drainage into spinal perimedullary veins and are classified further as spinal dAVFs.53
Borden et al51 classified dAVFs into three types based on patterns of venous drainage. Type I lesions drain antegrade directly into a major venous dural sinus. Type II lesions drain into the venous dural sinus with retrograde drainage into subarachnoid veins. Type III lesions drain retrograde into cortical veins. Types II and III are associated with significant venous hypertension and are considered at high risk for hemorrhage. We typically use the Borden classification system and refer to dAVFs using this classification in this chapter’s discussion.
♦ Natural History and Treatment Algorithm
The decision to treat dAVFs can depend on a several factors, including the patient’s symptoms, presence of hemorrhage, location of the fistula, its angiographic features, and changes in the lesion over time.39,40,42,49,51,54–56 Borden type I dAVFs are thought to have a benign natural history, with one series57 showing only one hemorrhage in 68 untreated patients followed for at least 27 months. However, type I lesions carry a 2% risk of progression to a higher grade.57,58 This progression is not always heralded by new symptoms.
Higher-grade dAVFs are considered more dangerous. Van Dijk et al59 followed 20 patients with type II or III dAVFs over a total of 87 patient-years, calculating an 8% risk of hemorrhage and 10% risk of death on an annual basis. This and other studies have justified the aggressive treatment of type II or III lesions, especially when symptomatic.56,60,61 Study of asymptomatic patients with high-risk angiographic features, such as cortical venous drainage (CVD) with distal sinus stenosis or deep venous drainage, demonstrated a high annualized risk of hemorrhage.59,62,63 However, recent natural history data from Strom et al64 of type II or III dAVFs demonstrated that lesions exhibiting asymptomatic CVD follow a more benign course than similar lesions with symptomatic CVD (1.4% versus 19% per year event rate). Similarly, Söderman et al60 followed 85 patients with dAVF and CVD and found the annual risk of hemorrhage varied depending on hemorrhage at presentation: 7.5% per year for patients presenting with hemorrhage, and only 1.5% per year for patients without. The presence of high-risk features requires careful consideration when determining treatment strategy.
Patients with low-risk dAVFs and no associated neurologic symptoms can be observed and should undergo regular angiographic follow-up. Low-risk dAVFs associated with flow-related headaches, orbital symptoms, or bruits that impair the quality of life may be treated with carotid compressive therapy45,65–68 or palliative transarterial embolization. The goal of treatment of high-risk dAVFs is complete obliteration; however, high-grade lesions with complex or inaccessible features are sometimes treated with surgical or endovascular disconnection of the cortical venous drainage.69,70 This leaves the fistula intact, converting a high-risk dAVF to a more benign lesion, although this outcome is not as favorable as complete resection or obliteration. As with cavernous malformations, treatment recommendations should be based on both clinical presentation and angiographic features.
♦ Surgical Approach and Microsurgical Technique
The key to successful surgical management of posterior fossa dAVFs is appropriate angiographic identification of the fistulous connection. Careful evaluation of the primary source on angiographic images enables the surgeon to identify the exact point at which arterial blood flow from the extracranial circulation enters the venous circulation. Knowing this exact location, the fistula can be localized within the posterior fossa (i.e., transverse-sigmoid junction, torcular Herophili, superior petrosal sinus, etc.). Once the location is determined, the appropriate surgical approach can be chosen. Almost all posterior fossa dAVFs can be accessed through a retrosigmoid, supratentorial-infratentorial, or lateral suboccipital craniotomy (Figs. 10.8 and 10.9).71
Intraoperatively, careful exploration allows the artery feeding the fistula to be identified. At this point, simple ligation of the fistula is performed with arteriovenous malformation (AVM) clips. Numerous tiny arterial feeding branches may be observed in the vicinity of the fistula. These branches may be ligated at surgery, but disruption of the main fistula is sufficient to obliterate the entire lesion.72,73 If multiple small-to-medium feeders that cannot be reached easily are present, preoperative transarterial embolization is helpful. When external ligation is not technically feasible, direct embolization of the fistula with muslin packing is a useful technique. Intraoperative angiography is extremely useful to confirm complete obliteration before closure. Close clinical and angiographic follow-up is warranted to check for recurrences.
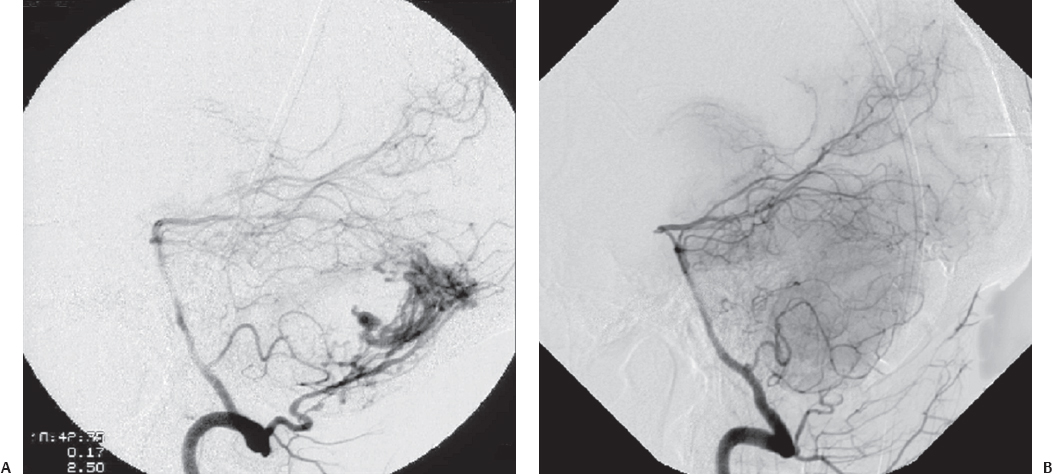
Fig. 10.8 (A) Torcular dural arteriovenous fistula (dAVF) approached via suboccipital craniotomy. (B) Postoperative obliteration of the dAVF by direct clipping of the fistulous connection. (Courtesy of Barrow Neurological Institute.)
♦ Endovascular Treatment
At our institution, endovascular treatment options for dAVFs are usually explored first. Careful patient selection for endovascular therapy is paramount. Even with contemporary techniques, specific angiographic features make some dAVFs better suited for stand-alone endovascular treatment and others better suited for combined endovascular, surgical, and/or radiosurgical treatment. If a fistula has normal anterograde sinus drainage, every effort must be made to maintain the patency of the parent sinus. Inadvertent occlusion of the parent dural sinus may be poorly tolerated and can lead to severe venous outflow obstruction and venous infarction. Caragine et al74 presented a series of patients with transverse-sigmoid junction dAVFs associated with a venous pouch parallel to the parent sinus. By recognizing this separate, parallel channel, the endovascular surgeon can safely embolize the venous pouch, preserve the parent sinus, and cure the patient.
Lesions with parent sinus occlusion distal to the fistula can be obliterated with coiling of the sinus at the fistulous connection because there is a lower risk of losing important distal venous drainage. Local venous anatomy must be delineated for each case before executing parent sinus embolization. Alternatively, if the venous route to the dAVF is blocked by occlusion or if there is direct communication between the dAVF and a cortical vein, it is usually preferable to surgically obliterate the fistula directly.75 In such cases intraoperative direct puncture embolization of the fistula is another option.76
Prior to the advent of nonadhesive liquid embolic agents (such as Onyx; see below), endovascular treatment of dAVFs typically involved staged transarterial embolization of dAVF feeders followed by transvenous coiling. Smaller feeding branches could be obliterated with liquid adhesives agents such as N-butyl cyanoacrylate (NBCA), whereas larger arterial feeders were left intact for “road-mapping” purposes and for angiographic verification that subsequent transvenous coiling or surgical ligation has obliterated the fistula. This technique is still commonly used as primary treatment in many centers and in cases where curative transarterial Onyx embolization is not feasible.77
Onyx (ev3 Endovascular, Plymouth, MN), a nonadhesive liquid embolic agent originally approved for the endovascular occlusion of arteriovenous malformations, is an emerging endovascular treatment for dAVFs. Onyx is cohesive, rather than adhesive, and is considered a more controllable and less rapid form of embolization compared with NBCA. Stiefel et al78 treated 28 patients with dAVFs of various grades, achieving angiographic cure in 21. Similar results were obtained by Cognard et al79 in their series of 30 patients. Several other series have described excellent short-and long-term cure rates with Onyx treatment of dAVF.80–82 Long-term angiographic and clinical follow-up of such treatments is needed to confirm the durable efficacy of Onyx embolization.
♦ Surgical Treatment
For those dAVFs where definitive endovascular occlusion cannot be safely obtained, surgical ligation is necessary. Similar to multimodality treatment in arteriovenous malformations,83 preoperative embolization of arterial connections, particularly in surgically inaccessible areas, can facilitate the surgeon’s task of dissecting and ligating the main fistula (Fig. 10.10). If a combined approach is warranted, embolization of arteries with significant scalp supply (superficial temporal, occipital, posterior auricular) is avoided to prevent possible skin necrosis after surgery.84 Instead, middle meningeal artery feeders are targeted and embolized.
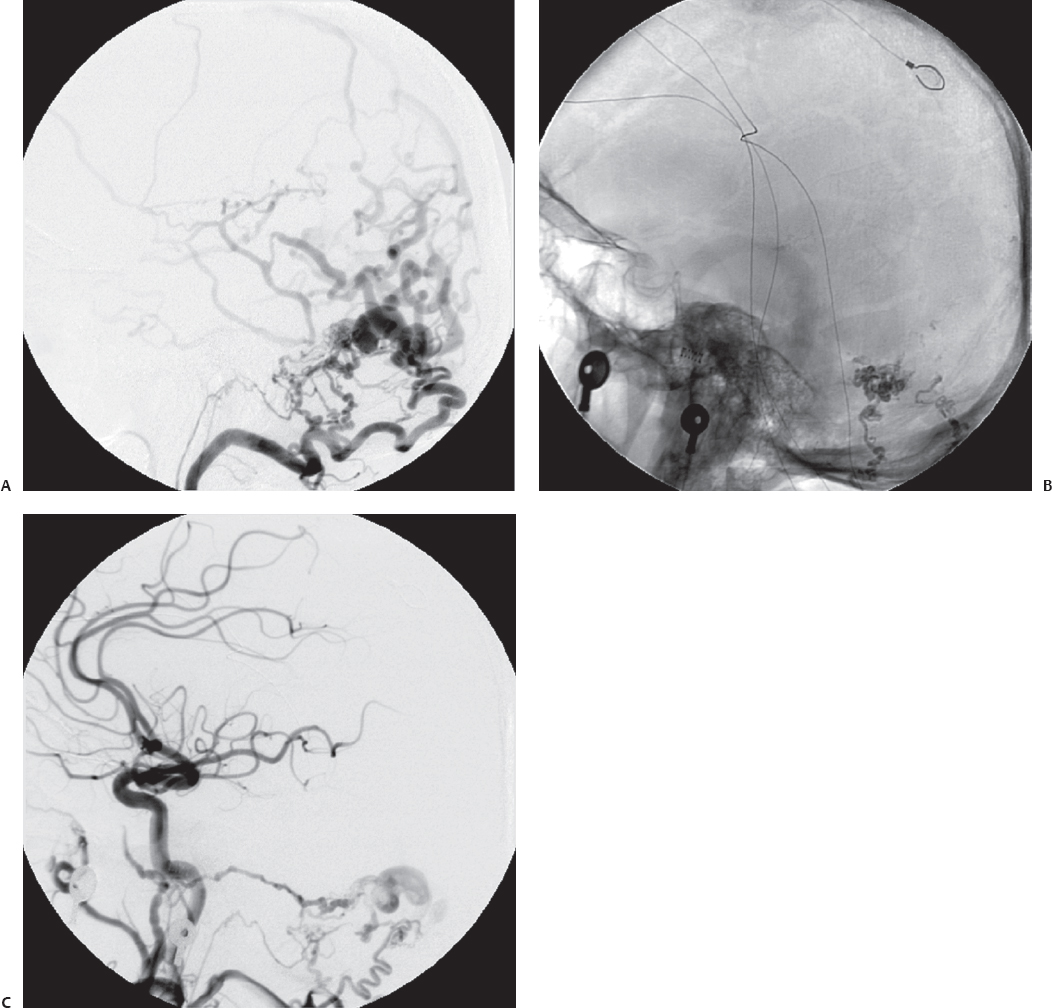
Fig. 10.9 Anteroposterior (A) and lateral (B) angiograms showing a tentorial dAVF. (C) Postoperative angiogram after retrosigmoid craniotomy and direct clipping of the fistulous connection shows excellent obliteration of the lesion. (Courtesy of Barrow Neurological Institute.)
Surgical obliteration of dAVFs has a high success rate. The best surgical approach is matched to the location of the lesion. Meticulous skeletonization of the associated dural sinus is required, including disconnection of feeders from the tentorium and falx where applicable. In 23 patients with highgrade dAVFs treated with surgery with or without preoperative embolization, Liu et al72 reported complete angiographic obliteration and no perioperative complications or further clinical events with up to 84 months of follow-up. Another series of 17 patients with high-grade dAVFs treated with surgery alone showed angiographic cure in 16.85 Collice et al86 described complete angiographic cure in 34 patients treated with surgery with or without embolization. Overall, surgery is considered a safe and effective treatment for dAVFs, particularly when embolization is not feasible or curative.
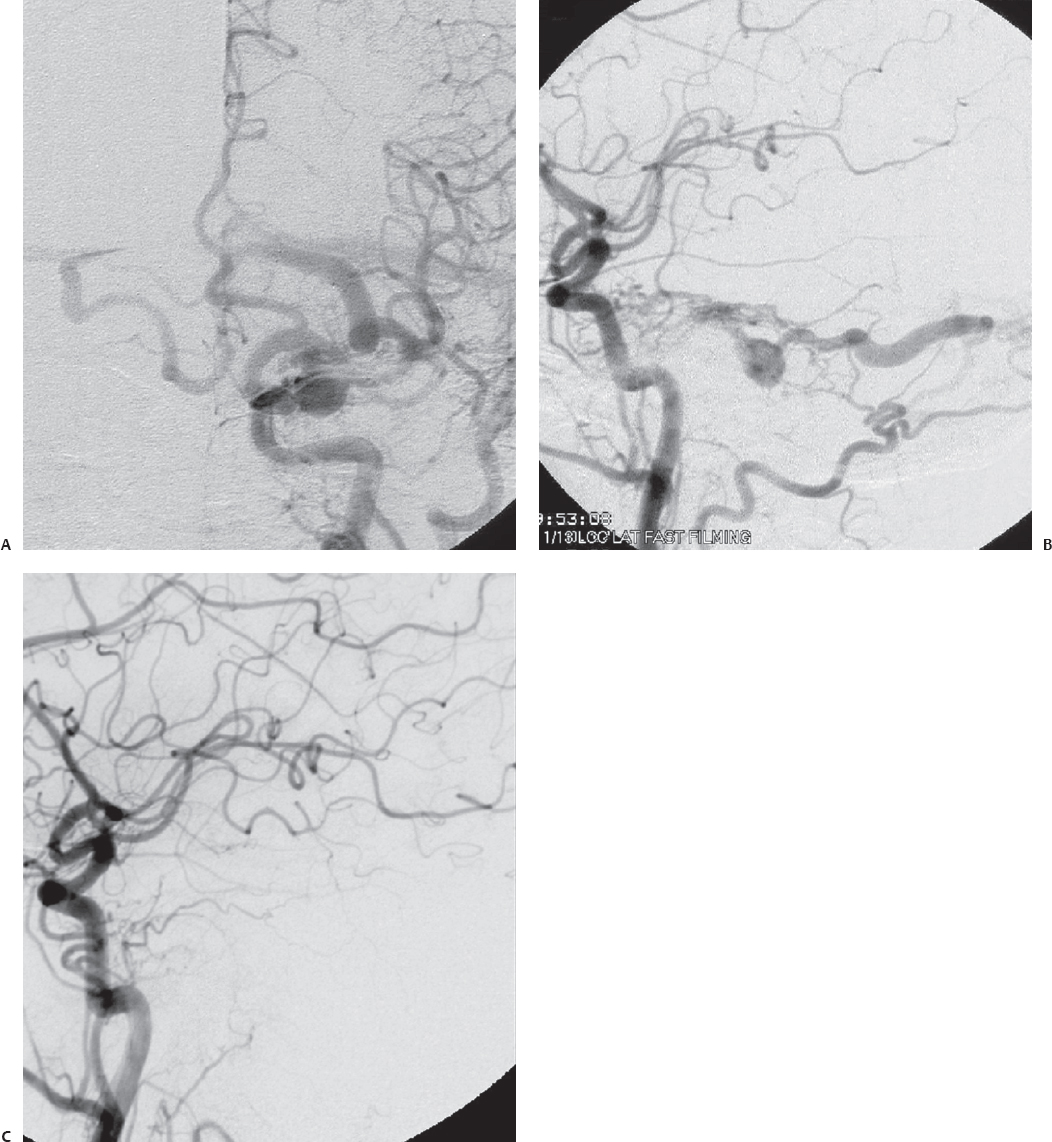
Fig. 10.10 (A) Left transverse-sigmoid sinus region dAVF with primary cortical venous drainage and obstruction of transverse sinus outflow. (B) Scout film after preoperative embolization. (C) Postembolization angiogram shows significant reduction of several of the occipital artery feeders. Residual feeders are present due to endovascular inaccessibility. (Courtesy of Barrow Neurological Institute.)
♦ Radiosurgery
Recent data have suggested that GKRS, either with or without tandem transarterial embolization, may provide therapeutic relief of symptoms and potentially promote the obliteration of fistulas.87–89 Pan et al87 reported that over a median follow-up of 19 months, 58% of patients were angiographically cured after GKRS at a dose of 16.5 to 19 Gy at the 50 to 70% isodose line. Friedman et al88 described 25 radiosurgery patients, 22 of whom also received transarterial embolization. Of these patients, 17 ultimately underwent angiographic follow-up, and 11 patients demonstrated “total or near total (>90%) obliteration.” All but one patient experienced immediate relief of symptoms. Söderman et al90 studied GKRS in 53 patients with dAVFs and reported an angiographic cure rate of 68% at 2 years. Wu et al91 studied GKRS in 81 patients with dAVFs and found complete or partial symptomatic relief in 75 after treatment. However, angiographic cure at 24 months was only 50% and was slightly less in patients with high-grade lesions. Although the clinical outcome of patients treated with GKRS seems to be better than the natural history of untreated patients, relief of symptoms is not directly comparable to a surgical or endovascular cure. Nonetheless, this modality is recognized as a useful adjuvant or stand-alone treatment of dAVFs. Posterior fossa dAVFs commonly recruit feeding arteries from petrosal and tentorial collaterals. The former typically derive from branches of the neuromeningeal branch of the ascending pharyngeal artery. These are usually unsafe for embolization due to well-known collateral branches to the cranial nerves. The latter can have significant contributions from the tentorial branch of the meningohypophyseal trunk. Typically, due to the high tortuosity of this vessel, embolization is not feasible. Therefore, GKRS is ideally suited for high-risk transpetrosal or skull-based dAVFs that cannot be safely treated with embolization due to potential collateral arterial feeders to the posterior circulation and cranial nerves or that are poorly accessible surgically.
References
1. McCormick WF, Hardman JM, Boulter TR. Vascular malformations (“angiomas”) of the brain, with special reference to those occurring in the posterior fossa. J Neurosurg 1968;28:241–251 PubMed
2. Frischer JM, Pipp I, Stavrou I, Trattnig S, Hainfellner JA, Knosp E. Cerebral cavernous malformations: congruency of histopathological features with the current clinical definition. J Neurol Neurosurg Psychiatry 2008;79:783–788 PubMed
3. Clatterbuck RE, Eberhart CG, Crain BJ, Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry 2001;71:188–192 PubMed
4. Fritschi JA, Reulen HJ, Spetzler RF, Zabramski JM. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir (Wien) 1994;130:35–46 PubMed
5. Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg 1995;83:820–824 PubMed
6. Simard JM, Garcia-Bengochea F, Ballinger WE Jr, Mickle JP, Quisling RG. Cavernous angioma: a review of 126 collected and 12 new clinical cases. Neurosurgery 1986;18:162–172 PubMed
7. Porter RW, Detwiler PW, Spetzler RF, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg 1999;90:50–58 PubMed
8. Deshmukh VR, Albuquerque FC, Zabramski JM, Spetzler RF. Surgical management of cavernous malformations involving the cranial nerves. Neurosurgery 2003;53:352–357, discussion 357 PubMed
9. Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg 1997;87:190–197 PubMed
10. Cantu C, Murillo-Bonilla L, Arauz A, Higuera J, Padilla J, Barinagarrementeria F. Predictive factors for intracerebral hemorrhage in patients with cavernous angiomas. Neurol Res 2005;27:314–318 PubMed
11. Labauge P, Brunereau L, Laberge S, Houtteville JP. Prospective follow-up of 33 asymptomatic patients with familial cerebral cavernous malformations. Neurology 2001;57:1825–1828 PubMed
12. Wang CC, Liu A, Zhang JT, Sun B, Zhao YL. Surgical management of brain-stem cavernous malformations: report of 137 cases. Surg Neurol 2003;59:444–454, discussion 454 PubMed
13. Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg 1991;75:709–714 PubMed
14. Robinson JR Jr, Awad IA, Magdinec M, Paranandi L. Factors predis posing to clinical disability in patients with cavernous malformations of the brain. Neurosurgery 1993;32:730–735, discussion 735–736 PubMed
15. Pozzati E, Giuliani G, Nuzzo G, Poppi M. The growth of cerebral cavernous angiomas. Neurosurgery 1989;25:92–97 PubMed
16. Barker FG II, Amin-Hanjani S, Butler WE, et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery 2001;49:15–24, discussion 24–25 PubMed
17. Ghogawala Z, Ogilvy CS. Intramedullary cavernous malformations of the spinal cord. Neurosurg Clin N Am 1999;10:101–111 PubMed
18. Kupersmith MJ, Kalish H, Epstein F, et al. Natural history of brainstem cavernous malformations. Neurosurgery 2001;48:47–53, discussion 53–54 PubMed
19. Brown AP, Thompson BG, Spetzler RF. The two-point method: Evaluating brainstem lesions. BNI Q 1996;12:20–24
20. de Oliveira JG, Lekovic GP, Safavi-Abbasi S, et al. Supracerebellar infratentorial approach to cavernous malformations of the brainstem: surgical variants and clinical experience with 45 patients. Neurosurgery 2010; 66:389–399 PubMed
21. Garrett M, Spetzler RF. Surgical treatment of brainstem cavernous malformations. Surg Neurol 2009;72(Suppl 2):S3–S9, discussion S9–S10 PubMed
22. Chen X, Weigel D, Ganslandt O, Buchfelder M, Nimsky C. Diffusion tensor imaging and white matter tractography in patients with brainstem lesions. Acta Neurochir (Wien) 2007;149:1117–1131, discussion 1131 PubMed
23. Romano A, D’Andrea G, Minniti G, et al. Pre-surgical planning and MR tractography utility in brain tumour resection. Eur Radiol 2009;19(12): 2798–2808.
24. Chen X, Weigel D, Ganslandt O, Fahlbusch R, Buchfelder M, Nimsky C. Diffusion tensor-based fiber tracking and intraoperative neuronavigation for the resection of a brainstem cavernous angioma. Surg Neurol 2007;68:285–291, discussion 291 PubMed
25. Recalde RJ, Figueiredo EG, de Oliveira E. Microsurgical anatomy of the safe entry zones on the anterolateral brainstem related to surgical approaches to cavernous malformations. Neurosurgery 2008;62(3, Suppl 1):9–15, discussion 15–17 PubMed
26. Rigamonti D, Spetzler RF. The association of venous and cavernous malformations. Report of four cases and discussion of the pathophysiological, diagnostic, and therapeutic implications. Acta Neurochir (Wien) 1988;92:100–105 PubMed
27. Dillon WP. Cryptic vascular malformations: controversies in terminology, diagnosis, pathophysiology, and treatment. AJNR Am J Neuroradiol 1997;18:1839–1846 PubMed
28. Steinberg GK, Chang SD, Gewirtz RJ, Lopez JR. Microsurgical resection of brainstem, thalamic, and basal ganglia angiographically occult vascular malformations. Neurosurgery 2000;46:260–270, discussion 270–271 PubMed
29. Amin-Hanjani S, Ogilvy CS, Candia GJ, Lyons S, Chapman PH. Stereotactic radiosurgery for cavernous malformations: Kjellberg’s experience with proton beam therapy in 98 cases at the Harvard Cyclotron. Neurosurgery 1998;42:1229–1236, discussion 1236–1238 PubMed
30. Ghannane H, Khalil T, Sakka L, Chazal J. Analysis of a series of cavernomas of the central nervous system: 39 non operated cases, 39 operated cases, 1 dead. Neurochirurgie 2007;53(2-3 Pt 2):217–222 PubMed
31. Bertalanffy H, Gilsbach JM, Eggert HR, Seeger W. Microsurgery of deep-seated cavernous angiomas: report of 26 cases. Acta Neurochir (Wien) 1991;108:91–99 PubMed
32. Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg 1997;87:267–274 PubMed
33. Chang SD, Levy RP, Adler JR Jr, Martin DP, Krakovitz PR, Steinberg GK. Stereotactic radiosurgery of angiographically occult vascular malformations: 14-year experience. Neurosurgery 1998;43:213–220, discussion 220–221 PubMed
34. Hasegawa T, McInerney J, Kondziolka D, Lee JY, Flickinger JC, Lunsford LD. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery 2002;50:1190–1197, discussion 1197–1198 PubMed
35. Pollock BE, Garces YI, Stafford SL, Foote RL, Schomberg PJ, Link MJ. Stereotactic radiosurgery for cavernous malformations. J Neurosurg 2000;93:987–991 PubMed
36. Lunsford LD, Khan AA, Niranjan A, Kano H, Flickinger JC, Kondziolka D. Stereotactic radiosurgery for symptomatic solitary cerebral cavernous malformations considered high risk for resection. J Neurosurg 2010; 113:23–29 PubMed
37. Awad IA, Barrow DL. Conceptual overview and management strategies. In: Awad IA, Barrow DL, eds. Dural Arteriovenous Malformations. Park Ridge, IL: AANS, 1993:231–241
38. Halbach VV, Higashida RT, Hieshima GB, Wilson CB, Hardin CW, Kwan E. Treatment of dural fistulas involving the deep cerebral venous system. AJNR Am J Neuroradiol 1989;10:393–399 PubMed
39. Lasjaunias P, Chiu M, ter Brugge K, Tolia A, Hurth M, Bernstein M. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg 1986;64:724–730 PubMed
40. Ishii K, Goto K, Ihara K, et al. High-risk dural arteriovenous fistulae of the transverse and sigmoid sinuses. AJNR Am J Neuroradiol 1987;8: 1113–1120 PubMed
41. Luessenhop AJ. Dural Arteriovenous Malformations. New York: McGraw-Hill, 2004
42. Malek AM, Halbach VV, Higashida RT, Phatouros CC, Meyers PM, Dowd CF. Treatment of dural arteriovenous malformations and fistulas. Neurosurg Clin N Am 2000;11:147–166, ix ix PubMed
43. McDougall CG, Halbach VV, Higashida RT. Treatment of dural arteriovenous fistulas. Neurosurg Q 1997;7:110–134
44. Du R, Binder DK, Halbach V, Fischbein N, Barbaro NM. Trigeminal neuralgia in a patient with a dural arteriovenous fistula in Meckel’s cave: case report. Neurosurgery 2003;53:216–221, discussion 221 PubMed
45. Shah SB, Lalwani AK, Dowd CF. Transverse/sigmoid sinus dural arteriovenous fistulas presenting as pulsatile tinnitus. Laryngoscope 1999; 109:54–58 PubMed
46. Herman JM, Spetzler RF, Bederson JB, Kurbat JM, Zabramski JM. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg 1995;83:539–545 PubMed
47. Awad IA, Little JR, Akarawi WP, Ahl J. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg 1990;72:839–850 PubMed
48. Awad IA. Intracranial dural arteriovenous malformations. In: Wilkins RH, Rengachary SS, eds. Neurosurgery. New York: McGraw-Hill, 1996: 2519–2528
49. Lalwani AK, Dowd CF, Halbach VV. Grading venous restrictive disease in patients with dural arteriovenous fistulas of the transverse/sigmoid sinus. J Neurosurg 1993;79:11–15 PubMed
50. Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995;194:671–680 PubMed
51. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995;82:166–179 PubMed
52. Djindjian R, Cophignon J, Théron J, Merland JJ, Houdart R. Embolization by superselective arteriography from the femoral route in neuroradiology. Review of 60 cases. 1. Technique, indications, complications. Neuroradiology 1973;6:20–26 PubMed
53. Kim LJ, Spetzler RF. Classification and surgical management of spinal arteriovenous lesions: arteriovenous fistulae and arteriovenous malformations. Neurosurgery 2006;59(5, Suppl 3):S195–S201, discussion S3–S13 PubMed
54. Tomak PR, Cloft HJ, Kaga A, Cawley CM, Dion J, Barrow DL. Evolution of the management of tentorial dural arteriovenous malformations. Neurosurgery 2003;52:750–760, discussion 760–762 PubMed
55. Halbach VV, Higashida RT, Hieshima GB, Mehringer CM, Hardin CW. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol 1989;10:385–392 PubMed
56. Kakarla UK, Deshmukh VR, Zabramski JM, Albuquerque FC, McDougall CG, Spetzler RF. Surgical treatment of high-risk intracranial dural arteriovenous fistulae: clinical outcomes and avoidance of complications. Neurosurgery 2007;61:447–457, discussion 457–459 PubMed
57. Satomi J, van Dijk JM, Terbrugge KG, Willinsky RA, Wallace MC. Benign cranial dural arteriovenous fistulas: outcome of conservative management based on the natural history of the lesion. J Neurosurg 2002; 97:767–770 PubMed
58. Cognard C, Houdart E, Casasco A, Gabrillargues J, Chiras J, Merland JJ. Long-term changes in intracranial dural arteriovenous fistulae leading to worsening in the type of venous drainage. Neuroradiology 1997; 39:59–66 PubMed
59. van Dijk JM, terBrugge KG, Willinsky RA, Wallace MC. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 2002;33:1233–1236 PubMed
60. Söderman M, Pavic L, Edner G, Holmin S, Andersson T. Natural history of dural arteriovenous shunts. Stroke 2008;39:1735–1739 PubMed
61. Duffau H, Lopes M, Janosevic V, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg 1999;90:78–84 PubMed
62. Davies MA, Ter Brugge K, Willinsky R, Wallace MC. The natural history and management of intracranial dural arteriovenous fistulae. Part 2: aggressive lesions. Interv Neuroradiol 1997;3:303–311 PubMed
63. Brown RD Jr, Flemming KD, Meyer FB, Cloft HJ, Pollock BE, Link ML. Natural history, evaluation, and management of intracranial vascular malformations. Mayo Clin Proc 2005;80:269–281 PubMed
64. Strom RG, Botros JA, Refai D, et al. Cranial dural arteriovenous fistulae: asymptomatic cortical venous drainage portends less aggressive clinical course. Neurosurgery 2009;64:241–247, discussion 247–248 PubMed
65. ApSimon HT, Ives FJ, Khangure MS. Cranial dural arteriovenous malformation and fistula. Radiological diagnosis and management. Review of thirty four patients. Australas Radiol 1993;37:2–25 PubMed
66. Halbach VV, Higashida RT, Hieshima GB, Reicher M, Norman D, Newton TH. Dural fistulas involving the cavernous sinus: results of treatment in 30 patients. Radiology 1987;163:437–442 PubMed
67. Higashida RT, Hieshima GB, Halbach VV, Bentson JR, Goto K. Closure of carotid cavernous sinus fistulae by external compression of the carotid artery and jugular vein. Acta Radiol Suppl 1986;369:580–583 PubMed
68. Halbach VV, Higashida RI, Hieshima GB, Dowd CF. Endovascular therapy of dural fistulas. In: Halbach VV, Vinuela F, Dion J, eds. Interventional Neuroradiology. New York: Raven Press, 1992:29–50
69. da Costa LB, Terbrugge K, Farb R, Wallace MC. Surgical disconnection of cortical venous reflux as a treatment for Borden type II dural arteriovenous fistulae. Acta Neurochir (Wien) 2007;149:1103–1108, discussion 1108 PubMed
70. van Dijk JM, TerBrugge KG, Willinsky RA, Wallace MC. Selective disconnection of cortical venous reflux as treatment for cranial dural arteriovenous fistulas. J Neurosurg 2004;101:31–35 PubMed
71. Lawton MT, Sanchez-Mejia RO, Pham D, Tan J, Halbach VV. Tentorial dural arteriovenous fistulae: operative strategies and microsurgical results for six types. Neurosurgery 2008;62(3, Suppl 1):110–124, discussion 124–125 PubMed
72. Liu JK, Dogan A, Ellegala DB, et al. The role of surgery for high-grade intracranial dural arteriovenous fistulas: importance of obliteration of venous outflow. J Neurosurg 2009;110(5):913–920 PubMed
73. Hoh BL, Choudhri TF, Connolly ES Jr, Solomon RA. Surgical management of high-grade intracranial dural arteriovenous fistulas: leptomeningeal venous disruption without nidus excision. Neurosurgery 1998;42:796–804, discussion 804–805 PubMed
74. Caragine LP, Halbach VV, Dowd CF, Ng PP, Higashida RT. Parallel venous channel as the recipient pouch in transverse/sigmoid sinus dural fistulae. Neurosurgery 2003;53:1261–1266, discussion 1266–1267 PubMed
75. Thompson BG, Doppman JL, Oldfield EH. Treatment of cranial dural arteriovenous fistulae by interruption of leptomeningeal venous drainage. J Neurosurg 1994;80:617–623 PubMed
76. Houdart E, Saint-Maurice JP, Chapot R, et al. Transcranial approach for venous embolization of dural arteriovenous fistulas. J Neurosurg 2002;97:280–286 PubMed
77. Guedin P, Gaillard S, Boulin A, et al. Therapeutic management of intracranial dural arteriovenous shunts with leptomeningeal venous drainage: report of 53 consecutive patients with emphasis on transarterial embolization with acrylic glue. J Neurosurg 2010;112:603–610 PubMed
78. Stiefel MF, Albuquerque FC, Park MS, Dashti SR, McDougall CG. Endovascular treatment of intracranial dural arteriovenous fistulae using Onyx: a case series. Neurosurgery 2009;65(6, Suppl):132–139, discussion 139–140 PubMed
79. Cognard C, Januel AC, Silva NA Jr, Tall P. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol 2008;29:235–241 PubMed
80. Panagiotopoulos V, Möller-Hartmann W, Asgari S, Sandalcioglu IE, Forsting M, Wanke I. Onyx embolization as a first line treatment for intracranial dural arteriovenous fistulas with cortical venous reflux. Rofo 2009;181:129–138 PubMed
81. Carlson AP, Taylor CL, Yonas H. Treatment of dural arteriovenous fistula using ethylene vinyl alcohol (onyx) arterial embolization as the primary modality: short-term results. J Neurosurg 2007;107:1120–1125 PubMed
82. Huang Q, Xu Y, Hong B, Li Q, Zhao W, Liu J. Use of onyx in the management of tentorial dural arteriovenous fistulae. Neurosurgery 2009;65: 287–292, discussion 292–293 PubMed
83. Natarajan SK, Ghodke B, Britz GW, Born DE, Sekhar LN. Multimodality treatment of brain arteriovenous malformations with microsurgery after embolization with onyx: single-center experience and technical nuances. Neurosurgery 2008;62:1213–1225, discussion 1225–1226 PubMed
84. Jankowitz BT, Vora N, Jovin T, Horowitz M. Ear necrosis resulting from the endovascular onyx-18 embolization of a dural arteriovenous fistula fed by the posterior auricular artery. J Neuroimaging 2009;19:259–262 PubMed
85. Ushikoshi S, Houkin K, Kuroda S, et al. Surgical treatment of intracranial dural arteriovenous fistulas. Surg Neurol 2002;57:253–261 PubMed
86. Collice M, D’Aliberti G, Arena O, Solaini C, Fontana RA, Talamonti G. Surgical treatment of intracranial dural arteriovenous fistulae: role of venous drainage. Neurosurgery 2000;47:56–66, discussion 66–67 PubMed
87. Pan DH, Chung WY, Guo WY, et al. Stereotactic radiosurgery for the treatment of dural arteriovenous fistulas involving the transversesigmoid sinus. J Neurosurg 2002;96:823–829 PubMed
88. Friedman JA, Pollock BE, Nichols DA, Gorman DA, Foote RL, Stafford SL. Results of combined stereotactic radiosurgery and transarterial embolization for dural arteriovenous fistulas of the transverse and sigmoid sinuses. J Neurosurg 2001;94:886–891 PubMed
89. Lewis AI, Tomsick TA, Tew JM Jr. Management of tentorial dural arteriovenous malformations: transarterial embolization combined with stereotactic radiation or surgery. J Neurosurg 1994;81:851–859 PubMed
90. Söderman M, Edner G, Ericson K, et al. Gamma knife surgery for dural arteriovenous shunts: 25 years of experience. J Neurosurg 2006;104: 867–875 PubMed
91. Wu HM, Pan DH, Chung WY, et al. Gamma Knife surgery for the management of intracranial dural arteriovenous fistulas. J Neurosurg 2006; 105(Suppl):43–51 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








