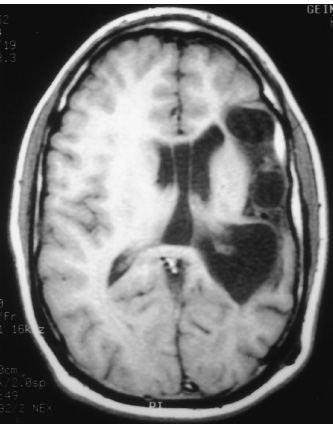
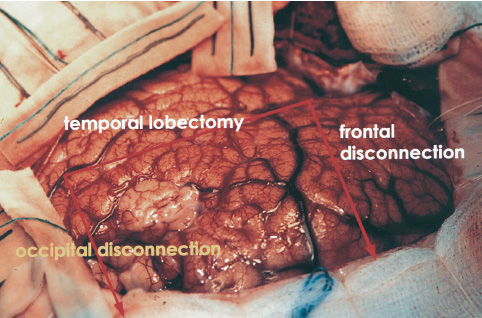
Walter Dandy completed the first hemispherectomy in 1928 when he attempted radical resection of a glioblastoma. Although surgical neurooncology containment was not achieved, the patient had acceptable postoperative neurologic function, and the foundation for anatomic resection of the cerebral hemisphere was introduced. In subsequent years, McKenzie (1938) and Krynauw (1950) reported the use of hemispherectomy in children with congenital hemiparesis who suffered intractable seizures. There was widespread acceptance of anatomic resection for seizure control until late hemorrhagic complications appeared, 3 to 20 years postoperatively. The mortality of chronic superficial hemosiderosis approached 33% in some series. Numerous modifications of Dandy’s surgical technique have been made to reduce the morbidity and long-term complications of hemispherectomy without reducing the long-term seizure control. Refinements have included the Adam’s technique of suturing the dura to the falx, which reduced the subdural cavity in exchange for a large epidural space, and early postoperative spinal fluid shunting. After observing that hemorrhagic complications did not occur following multiple lobectomies, Rasmussen (1983) developed the now widely accepted technique of functional hemispherectomy. After temporal lobectomy and wide resection of rolandic cortex into the ventricle, cortical incisions were extended to the midline, disconnecting broad polar blocks of the frontal and occipital lobes, which retained their blood supply. Other modifications to hemispherectomy have included hemi-decortication and hemicorticectomy. The technical evolution in hemispherectomy has been toward maximum conservation of cortical tissue. More recent refinements include intraventricular deafferentation procedures disconnecting large regions of cortex through infrasylvian and suprasylvian cortical windows. Endoscopic deafferentation in patients with congential porencephaly/infarct syndromes is on the horizon. We are pursuing the development of this technique in our laboratory. Children appear quite sensitive to surgical manipulation within the body of the lateral ventricles; postoperative fever, lethargy, and inflammatory ventriculitis syndromes are common. We have used intraoperative ultrasound to tailor the frontal and occipital polar disconnections of modified hemispherectomy (MH) to avoid entrance into the ventricle and substituted central topectomy for rolandic resection. This chapter reviews the surgical techniques of hemispherectomy and highlights the use of intraoperative ultrasound to refine functional hemispherectomy resection. Children with intractable seizures are the best candidates for hemispherectomy. Generalized seizures are among the most common semiology, but complex partial, infantile spasms or jacksonian motor seizures may be encountered. The frequency of daily ictal events may range from several to 30 or more seizures. Seizures commonly begin in the first few months of life and are resistant to many anticonvulsant medications. In nearly all candidates for hemispherectomy, fine-dexterity hand movements are compromised. In select cases of patients suffering from Rasmussen’s encephalitis with progressive hemiparesis, hand dexterity and strength loss may be acceptable sacrifices for seizure control. The etiology of intractable seizures in candidates for hemispherectomy includes hemispheric dysplasia, Rasmussen’s encephalitis, cerebral hemorrhage or infarction, hemimegalencephaly, Sturge-Weber syndrome, schizencephaly, and porencephaly syndromes. Multiplanar magnetic resonance imaging (MRI) is the neuroradiological imaging study of choice and readily demonstrates the anatomic malformation of the affected cerebral hemisphere. The most common findings include encephalomalacia with an enlarged ipsilateral ventricle (Fig. 24–1). Other findings may include cortical dysplasias, vascular malformation, or schizencephaly. Single-positron emission computed tomography (SPECT) or PET identifies zones of abnormal cerebral blood flow and metabolism isolated within the involved cerebral hemisphere. Preoperative evaluation is completed by the members of an integrated pediatric epilepsy program, including pediatric neurosurgeons, neurologists, and neuropsychologists. Multiple outpatient and video-telemetric electroencephalographic recordings are made to isolate ictal epileptiform discharges to a single cerebral hemisphere. Neuropsychology and Wada testing are completed in each patient older than 5 years of age to demonstrate that the normal cerebral hemisphere has capable memory and eloquent function. Intracarotid amobarbital injection of the effected cerebral hemisphere is helpful in predicting postoperative contralateral leg function. In children too young to complete language localization studies, left hemi-spherectomy can be performed with confidence that capable language dominance will evolve within the right cerebral hemisphere. FIGURE 24–1. Preoperative axial T1 MRI sequence of an 18-year-old man with congenital right hemiparesis and intractable seizures. There is broad left middle cerebral territory infarction and mild ex vacuo left ventricular dilation. Dexamethasone is started perioperatively (1 mg/kg daily up to 24 mg/day), and two units of donor directed packed red blood cells are prepared. The patient’s usual anticonvulsants are given on the morning of surgery and are continued postoperatively for 1 year. In the first few days following surgery, drug levels can fluctuate widely and are closely monitored until corticosteroids are discontinued on the fifth postoperative day. Children completing hemispherectomy do not have elevated intracranial pressure. Unlike patients with tumors or intracranial hemorrhage who have marginal cerebral perfusion, candidates for hemispherectomy are typically healthy and have normal cardiac indices. Deliberate volume expansion is well tolerated, and anticipation of blood loss during hemispherectomy warrants avoidance of hypovolemia. Mild hemodilution with 20 mL/kg of isotonic saline diminishes red blood cell loss during intraoperative bleeding and limits the volume of transfusion. Initial blood loss is supported with isotonic crystalloid solutions and then is replaced with donor-directed packed red blood cells when intraoperative hemoglobin drops below 7.5 g/dL. Cerebral relaxation is achieved with mild hyperven-tilation; arterial pCO2 is maintained at 28 to 30 mm Hg until the temporal lobectomy and cortical disconnections are completed. The remainder of the hemispherectomy is completed with eucarbia. Diuresis with mannitol or Lasix is generally not necessary to augment cerebral relaxation, even during resection of an enlarged hemi-megalencephalic hemisphere. Avoidance of hypothermia through the use of warmed, humidified inspired air and warming circuits for fluids and blood products is critical to preserve platelet agglutination and avoid coagulopathy. A constant dialogue with the anesthesiologist is important in minimizing delays in fluid or blood product replacement. After induction of general anesthesia, bladder, arterial, and central venous catheters are placed, and the patient is positioned supine. The ipsilateral shoulder is elevated, and the head is rotated sufficient for midline alignment in a neutral plane. We avoid the use of skull fixation, preferring support on a padded Mayfield horseshoe headrest, which allows intraoperative rotation or manipulation of the head for maximum visibility at the midline. A path of hair is shaved along the Dandy incision, and the scalp is prepared with Betadine scrub, alcohol, and Betadine solution. Intravenous nafcillin (50 mg/kg up to l.5 g) and gentamycin (l mg/kg) of body weight are administered before the skin incision is made, and nafcillin is continued every 6 hours for 24 hours following surgery. In the patient with a penicillin allergy, we substitute vancomycin, l0 mg/kg, slowly infused over 1 hour. In most cases of hemispherectomy, the lateral ventricle of the affected cerebral hemisphere is enlarged. We mark skin coordinates and use intraoperative ultrasound to guide ventriculostomy placement if necessary to enhance cerebral relaxation. This is rarely necessary during modified or ultrasound-guided hemispherectomy because entrance within the temporal horn of the ventricle during temporal lobectomy drains a moderate volume of spinal fluid. The temporalis muscle is divided along the path of the Dandy skin incision and elevated along a subperiosteal plane. A frontotemporal-parietal bone flap is fashioned with a high-speed craniotome to within 0.5 cm of the midline and of the floor of the middle cranial fossa. Additional exposure of the anterior inferior temporal lobe is achieved with rongeur resection of the sphenoid bone. Frontal exposure continues across the sphenoid wing along the floor of the anterior cranial fossa. The posterior margin of the bone flap extends about 4 cm behind the pinna. Epidural tack-up sutures are positioned circumferentially along the margin of the bone flap. Based on the midline, the dura is opened along the perimeter of the exposure and, to minimize shrinkage, reflected under tension within moistened Telfa strips (Fig. 24–2). Beginning within the superior temporal gyrus, an enbloc resection of the lateral temporal gyri is completed toward the collateral sulcus. In a coronal plane, within the inferior margin of the middle temporal gyrus, ultrasound guides entrance into the temporal horn (Figs. 24–3 and 24–4); the hippocampus then is uncovered from the pes toward the atria. We resect the mesial temporal structures, including the amygdala, hippocampus and the uncus with the CUSA using a subpial technique. After entering the choroid plexus, penetrating branches of the anterior choroidal artery may also supply the posterior limb of the internal capsule and the adjacent thalamus. Bipolar coagulation of the choroidal fissure is avoided because injury of these vessels can lead to flaccid hemiparesis or thalamic infarction. Every effort is made to preserve the mesial pia, protecting the cerebral peduncle, the third cranial nerve, and the perforators and larger vessels about the circle of Willis.
SURGICAL INDICATIONS AND PREOPERATIVE EVALUATION
PREOPERATIVE MANAGEMENT
INTRAOPERATIVE TECHNIQUES
Anesthetic Techniques and Positioning
Ultrasound-Guided Functional Hemispherectomy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


